Abstract
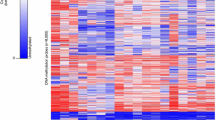
Gliomatosis cerebri (GC) is presently considered a distinct astrocytic glioma entity according to the WHO classification for CNS tumors. It is characterized by widespread, typically bilateral infiltration of the brain involving three or more lobes. Genetic studies of GC have to date been restricted to the analysis of individual glioma-associated genes, which revealed mutations in the isocitrate dehydrogenase 1 (IDH1) and tumor protein p53 (TP53) genes in subsets of patients. Here, we report on a genome-wide analysis of DNA methylation and copy number aberrations in 25 GC patients. Results were compared with those obtained for 105 patients with various types of conventional, i.e., non-GC gliomas including diffuse astrocytic gliomas, oligodendrogliomas and glioblastomas. In addition, we assessed the prognostic role of methylation profiles and recurrent DNA copy number aberrations in GC patients. Our data reveal that the methylation profiles in 23 of the 25 GC tumors corresponded to either IDH mutant astrocytoma (n = 6), IDH mutant and 1p/19q codeleted oligodendroglioma (n = 5), or IDH wild-type glioblastoma including various molecular subgroups, i.e., H3F3A-G34 mutant (n = 1), receptor tyrosine kinase 1 (RTK1, n = 4), receptor tyrosine kinase 2 (classic) (RTK2, n = 2) or mesenchymal (n = 5) glioblastoma groups. Two tumors showed methylation profiles of normal brain tissue due to low tumor cell content. While histological grading (WHO grade IV vs. WHO grade II and III) was not prognostic, the molecular classification as classic/RTK2 or mesenchymal glioblastoma was associated with worse overall survival. Multivariate Cox regression analysis revealed MGMT promoter methylation as a positive prognostic factor. Taken together, DNA-based large-scale molecular profiling indicates that GC comprises a genetically and epigenetically heterogeneous group of diffuse gliomas that carry DNA methylation and copy number profiles closely matching the common molecularly defined glioma entities. These data support the removal of GC as a distinct glioma entity in the upcoming revision of the WHO classification.



Similar content being viewed by others
Abbreviations
- A_IDH:
-
Astrocytoma IDH mutant
- CIMP:
-
CpG island methylator phenotype
- CNS:
-
Central nervous system
- EGFR:
-
Epidermal growth factor receptor
- GBM_G34:
-
Glioblastoma WHO grade IV H3F3A-G34 mutant subgroup
- GBM_RTK1:
-
Glioblastoma WHO grade IV receptor tyrosine kinase 1 subgroup
- GBM_RTK2:
-
Glioblastoma WHO grade IV receptor tyrosine kinase 2 (classic) subgroup
- GBM_mes:
-
Glioblastoma WHO grade IV mesenchymal subgroup
- GC:
-
Gliomatosis cerebri
- H3F3A:
-
H3 histone, family 3A
- IDH:
-
Isocitrate dehydrogenase
- MGMT:
-
O6-Methylguanine DNA methyltransferase
- mOS:
-
Median overall survival
- MRI:
-
Magnetic resonance imaging
- O_IDH:
-
Oligodendroglioma IDH mutant and 1p/19q codeleted
- TP53:
-
Tumor protein p53
- WHO:
-
World Health Organization
References
Bady P, Sciuscio D, Diserens AC, Bloch J, van den Bent MJ, Marosi C et al (2012) MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol 124:547–560
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477
Network Cancer Genome Atlas Research, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498
Desestret V, Ciccarino P, Ducray F, Crinière E, Boisselier B, Labussière M et al (2011) Prognostic stratification of gliomatosis cerebri by IDH1 R132H and INA expression. J Neurooncol 105:219–224
D’Urso OF, D’Urso PI, Marsigliante S (2009) Correlative analysis of gene expression profile and prognosis in patients with gliomatosis cerebri. Cancer 115:3749–3757
Fuller GN, Kros JM (2007) Gliomatosis cerebri. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO Classification of Tumours of the Central Nervous System. International Agency for Research on Cancer, Lyon, pp 50–52
Glas M, Bähr O, Felsberg J, Rasch K, Wiewrodt D, Schabet M et al (2011) NOA-05 phase 2 trial of procarbazine and lomustine therapy in gliomatosis cerebri. Ann Neurol 70:445–453
Herrlinger U (2012) Gliomatosis cerebri. Handb Clin Neurol 105:507–515
Herrlinger U, Felsberg J, Küker W, Bornemann A, Plasswilm L, Knobbe CB et al (2002) Gliomatosis cerebri: molecular pathology and clinical course. Ann Neurol 52:390–399
Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R, Cavalli FM et al (2013) Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathol 125:913–916
Kaloshi G, Everhard S, Laigle-Donadey F, Marie Y, Navarro S, Mokhtari K et al (2008) Genetic markers predictive of chemosensitivity and outcome in gliomatosis cerebri. Neurology 70:590–595
Korshunov A, Ryzhova M, Hovestadt V, Bender S, Sturm D, Capper D et al (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129:669–678
Mawrin C, Kirches E, Schneider-Stock R, Boltze C, Vorwerk CK, von Mawrin A et al (2006) Alterations of cell cycle regulators in gliomatosis cerebri. J Neurooncol 72:115–122
Mawrin C, Kirches E, Schneider-Stock R, Scherlach C, Vorwerk C, Von Deimling A et al (2003) Analysis of TP53 and PTEN in gliomatosis cerebri. Acta Neuropathol 105:529–536
Pajtler KW, Witt H, Sill M, Jones DT, Hovestadt V, Kratochwil F et al (2015) Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27:728–743
Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C et al (2015) Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol 130:407–417
Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A et al (2015) IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol 129:867–873
Seiz M, Tuettenberg J, Meyer J, Essig M, Schmieder K, Mawrin C et al (2010) Detection of IDH1 mutations in gliomatosis cerebri, but only in tumors with additional solid component: evidence for molecular subtypes. Acta Neuropathol 120:261–267
Sturm D, Witt H, Hovestadt V, Khuong-Quang DA, Jones DT, Konermann C et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Suzuki H, Aoki K, Chiba K, Sato Y, Shiozawa Y, Shiraishi Y et al (2015) Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47:458–468
Taillibert S, Chodkiewicz C, Laigle-Donadey F, Napolitano M, Cartalat-Carel S, Sanson M (2006) Gliomatosis cerebri: a review of 296 cases from the ANOCEF database and the literature. J Neurooncol 76:201–205
Ware ML, Hirose Y, Scheithauer BW, Yeh RF, Mayo MC, Smith JS et al (2007) Genetic aberrations in gliomatosis cerebri. Neurosurgery 60:150–158
Wiestler B, Capper D, Sill M, Jones DT, Hovestadt V, Sturm D et al (2014) Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol 128:561–571
Wiestler B, Capper D, Hovestadt V, Sill M, Jones DT, Hartmann C et al (2014) Assessing CpG island methylator phenotype, 1p/19q codeletion, and MGMT promoter methylation from epigenome-wide data in the biomarker cohort of the NOA-04 trial. Neuro Oncol 16:1630–1638
Acknowledgments
The authors would like to thank the German Cancer Research Center Genomics and Proteomics Core Facility (DKFZ GPCF) and Britta Friedensdorf, Düsseldorf, for excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the German Cancer Consortium (DKTK) joint funding project ‘Next Generation Molecular Diagnostics of Malignant Gliomas’.
Conflict of interest
UH has received advisory board honoraria from Roche, Mundipharma and Novocure, speakers honoraria from Roche, Medac and Mundipharma, travel reimbursement from Roche and Medac, grant support from Roche; MG has received advisory board honoraria from Roche and Mundipharma, speakers honoraria from Novartis, Roche and UCB, travel reimbursement from Roche and Medac; JPS has received advisory board honoraria from Roche and Mundipharma, speakers honoraria and travel reimbursement from Medac and Roche, grant support from Merck; MW has received advisory board honoraria from Celldex, Immunocellular Therapeutics, Magforce, Isarna MSD, Merck, Northwest Biotherapeutics, Novocure, Pfizer, Roche and Teva, Grant support from Acceleron, Actelion, Alpinia Institute, Bayer, Isarna, MSD, Merck, Novocure, PIQUR and Roche. All other authors do not have any conflict of interest.
Additional information
U. Herrlinger, D. T. W. Jones and M. Glas share first authorship.
M. Weller and G. Reifenberger share last authorship.
Rights and permissions
About this article
Cite this article
Herrlinger, U., Jones, D.T.W., Glas, M. et al. Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 131, 309–319 (2016). https://doi.org/10.1007/s00401-015-1495-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1495-z