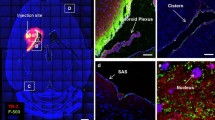
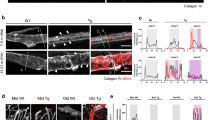
Abstract
The interstitial fluid (ISF) drainage pathway has been hypothesized to underlie the clearance of solutes and metabolites from the brain. Previous work has implicated the perivascular spaces along arteries as the likely route for ISF clearance; however, it has never been demonstrated directly. The accumulation of amyloid β (Aβ) peptides in brain parenchyma is one of the pathological hallmarks of Alzheimer disease (AD), and it is likely related to an imbalance between production and clearance of the peptide. Aβ drainage along perivascular spaces has been postulated to be one of the mechanisms that mediate the peptide clearance from the brain. We therefore devised a novel method to visualize solute clearance in real time in the living mouse brain using laser guided bolus dye injections and multiphoton imaging. This methodology allows high spatial and temporal resolution and revealed the kinetics of ISF clearance. We found that the ISF drains along perivascular spaces of arteries and capillaries but not veins, and its clearance exhibits a bi-exponential profile. ISF drainage requires a functional vasculature, as solute clearance decreased when perfusion was impaired. In addition, reduced solute clearance was observed in transgenic mice with significant vascular amyloid deposition; we suggest the existence of a feed-forward mechanism, by which amyloid deposition promotes further amyloid deposition. This important finding provides a mechanistic link between cerebrovascular disease and Alzheimer disease and suggests that facilitation of Aβ clearance along the perivascular pathway should be considered as a new target for therapeutic approaches to Alzheimer disease and cerebral amyloid angiopathy.





Similar content being viewed by others
References
Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552. doi:10.1016/j.neuint.2003.11.006
Attems J, Jellinger KA, Lintner F (2005) Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol 110:222–231. doi:10.1007/s00401-005-1064-y
Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, Zlokovic BV (2007) Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 27:909–918. doi:10.1038/sj.jcbfm.9600419
Bradbury MW, Cserr HF, Westrop RJ (1981) Drainage of cerebral interstitial fluid into deep cervical lymph of the rabbit. Am J Physiol 240:F329–F336
Burns EM, Kruckeberg TW, Gaetano PK (1981) Changes with age in cerebral capillary morphology. Neurobiol Aging 2:283–291
Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO (2008) Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34:131–144. doi:10.1111/j.1365-2990.2007.00926.x
Casley-Smith JR, Foldi-Borsok E, Foldi M (1976) The prelymphatic pathways of the brain as revealed by cervical lymphatic obstruction and the passage of particles. Br J Exp Pathol 57:179–188
Cirrito JR, May PC, O’Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM (2003) In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J Neurosci Off J Soc Neurosci 23:8844–8853
Datta S, Satten E (2005) Rank-sum tests for clustered data. J Am Stat Assoc 100:908–915. doi:10.1198/016214504000001583
DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM (2002) Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science 295:2264–2267. doi:10.1126/science.1067568
Dietrich WD, Prado R, Halley M, Watson BD (1993) Microvascular and neuronal consequences of common carotid artery thrombosis and platelet embolization in rats. J Neuropathol Exp Neurol 52:351–360
Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G (2005) Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimer’s Dis JAD 7:221–232 discussion 255–262
Garcia-Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C, Frosch MP, Greenberg SM, Bacskai BJ (2011) Cerebrovascular lesions induce transient beta-amyloid deposition. Brain J Neurol 134:3697–3707. doi:10.1093/brain/awr300
Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP (2006) Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol Dis 24:516–524. doi:10.1016/j.nbd.2006.08.017
Greenberg SM, Gurol ME, Rosand J, Smith EE (2004) Amyloid angiopathy-related vascular cognitive impairment. Stroke J Cereb Circ 35:2616–2619. doi:10.1161/01.STR.0000143224.36527.44
Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO (2011) Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol 121:431–443. doi:10.1007/s00401-011-0801-7
Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y (2009) Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol 66:343–348. doi:10.1001/archneur.66.3.343
Hrabetova S, Nicholson C (2007) Biophysical properties of brain extracellular space explored with ion-selective microelectrodes, integrative optical imaging and related techniques. In: Michael AC, Borland LM (eds) Electrochemical methods for neuroscience, City
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Trans Med 4:147ra111. doi:10.1126/scitranslmed.3003748
Iwata N, Higuchi M, Saido TC (2005) Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther 108:129–148. doi:10.1016/j.pharmthera.2005.03.010
Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR (2001) Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng 17:157–165
Kalaria RN (1996) Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther 72:193–214
Kalaria RN (2000) The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging 21:321–330
Kleinfeld D, Mitra PP, Helmchen F, Denk W (1998) Fluctuations and stimulus-induced changes in blood flow observed in individual capillaries in layers 2 through 4 of rat neocortex. Proc Natl Acad Sci USA 95:15741–15746
Klunk WE, Bacskai BJ, Mathis CA, Kajdasz ST, McLellan ME, Frosch MP, Debnath ML, Holt DP, Wang Y, Hyman BT (2002) Imaging Abeta plaques in living transgenic mice with multiphoton microscopy and methoxy-X04, a systemically administered Congo red derivative. J Neuropathol Exp Neurol 61:797–805
Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR (2008) AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging 29:1587–1590. doi:10.1016/j.neurobiolaging.2007.03.008
Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL (1994) High-flow microinfusion: tissue penetration and pharmacodynamics. Am J physiol 266:R292–R305
Neuropathology Group. Medical Research Council Cognitive F, Aging S (2001) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology group of the medical research council cognitive function and ageing study (MRC CFAS). Lancet 357:169–175
Nicholson C (1985) Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res 333:325–329
Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, Kitamura A, Washida K, Yamada M, Ito H, Tomimoto H, Takahashi R, Ihara M (2012) Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 123:381–394. doi:10.1007/s00401-011-0925-9
Paulson OB, Waldemar G, Schmidt JF, Strandgaard S (1989) Cerebral circulation under normal and pathologic conditions. Am J Cardiol 63:2C–5C
Robbins EM, Betensky RA, Domnitz SB, Purcell SM, Garcia-Alloza M, Greenberg C, Rebeck GW, Hyman BT, Greenberg SM, Frosch MP, Bacskai BJ (2006) Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer disease. J Neurosci Off J Soc Neurosci 26:365–371. doi:10.1523/JNEUROSCI.3854-05.2006
Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV (2000) Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Investig 106:1489–1499. doi:10.1172/JCI10498
Shih AY, Blinder P, Tsai PS, Friedman B, Stanley G, Lyden PD, Kleinfeld D (2013) The smallest stroke: occlusion of one penetrating vessel leads to infarction and a cognitive deficit. Nat Neurosci 16:55–63. doi:10.1038/nn.3278
Shin HK, Jones PB, Garcia-Alloza M, Borrelli L, Greenberg SM, Bacskai BJ, Frosch MP, Hyman BT, Moskowitz MA, Ayata C (2007) Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain J Neurol 130:2310–2319. doi:10.1093/brain/awm156
Skoch J, Hickey GA, Kajdasz ST, Hyman BT, Bacskai BJ (2005) In vivo imaging of amyloid-beta deposits in mouse brain with multiphoton microscopy. Methods Mol Biol 299:349–363
Smith EE, Vijayappa M, Lima F, Delgado P, Wendell L, Rosand J, Greenberg SM (2008) Impaired visual evoked flow velocity response in cerebral amyloid angiopathy. Neurology 71:1424–1430. doi:10.1212/01.wnl.0000327887.64299.a4
Soontornniyomkij V, Lynch MD, Mermash S, Pomakian J, Badkoobehi H, Clare R, Vinters HV (2010) Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol 20:459–467. doi:10.1111/j.1750-3639.2009.00322.x
Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF (1984) Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246:F835–F844
Utter S, Tamboli IY, Walter J, Upadhaya AR, Birkenmeier G, Pietrzik CU, Ghebremedhin E, Thal DR (2008) Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol 67:842–856. doi:10.1097/NEN.0b013e3181836a71
Vinters HV (1987) Cerebral amyloid angiopathy. A critical review. Stroke J Cereb Circ 18:311–324
Viswanathan A, Greenberg SM (2011) Cerebral amyloid angiopathy in the elderly. Ann Neurol 70:871–880. doi:10.1002/ana.22516
Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD (1985) Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol 17:497–504. doi:10.1002/ana.410170513
Weed LH (1914) Studies on Cerebro-Spinal Fluid. No. III : the pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. J Med Res 31:51–91
Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE (1998) Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol 153:725–733
Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA (2007) Relation of cognitive activity to risk of developing Alzheimer disease. Neurology 69:1911–1920. doi:10.1212/01.wnl.0000271087.67782.cb
Wisniewski HM, Kozlowski PB (1982) Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT). Ann N Y Acad Sci 396:119–129
Woollam DH, Millen JW (1955) The perivascular spaces of the mammalian central nervous system and their relation to the perineuronal and subarachnoid spaces. J Anat 89:193–200
Yamaguchi H, Yamazaki T, Lemere CA, Frosch MP, Selkoe DJ (1992) Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer’s disease. An immunoelectron microscopic study. Am J Pathol 141:249–259
Zhang ET, Richards HK, Kida S, Weller RO (1992) Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol 83:233–239
Zhang S, Murphy TH (2007) Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol 5:e119. doi:10.1371/journal.pbio.0050119
Acknowledgments
We wish to express our gratitude to Dr. K. V. Kuchibhotla, Dr. A. Serrano-Pozo and Dr. T. L. Spires-Jones for fruitful discussions and careful reading and editing of the manuscript. This work was supported by the National Institute of Health (Grant No. EB000768).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arbel-Ornath, M., Hudry, E., Eikermann-Haerter, K. et al. Interstitial fluid drainage is impaired in ischemic stroke and Alzheimer’s disease mouse models. Acta Neuropathol 126, 353–364 (2013). https://doi.org/10.1007/s00401-013-1145-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1145-2