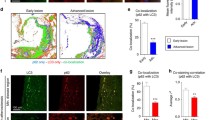
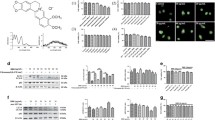
Abstract
Autophagy is a reparative, life-sustaining process by which cytoplasmic components are sequestered in double membrane vesicles and degraded upon fusion with lysosomal compartments. Mice with a macrophage-specific deletion of the essential autophagy gene Atg5 develop plaques with increased apoptosis and oxidative stress as well as enhanced plaque necrosis. This finding indicates that basal autophagy in macrophages is anti-apoptotic and present in atherosclerotic plaques to protect macrophages against various atherogenic stressors. However, autophagy is impaired in advanced stages of atherosclerosis and its deficiency promotes atherosclerosis in part through activation of the inflammasome. Because basal autophagy can be intensified selectively in macrophages by specific drugs such as mammalian target of rapamycin (mTOR) inhibitors or Toll-like receptor 7 (TLR7) ligands, these drugs were recently tested as potential plaque stabilizing compounds. Stent-based delivery of the mTOR inhibitor everolimus promotes a stable plaque phenotype, whereas local administration of the TLR7 ligand imiquimod stimulates inflammation and plaque progression. Therefore, more drugs capable of inducing autophagy should be tested in plaque macrophages to evaluate the feasibility of this approach. Given that drug-induced macrophage autophagy is associated with pro-inflammatory responses due to cytokine release, induction of postautophagic necrosis or activation of phagocytes after clearance of the autophagic corpse, cotreatment with anti-inflammatory compounds may be required. Overall, this review highlights the pros and cons of macrophage autophagy as a drug target for plaque stabilization.





Similar content being viewed by others
References
Alinari L, Baiocchi RA, Praetorius-Ibba M (2012) FTY720-induced blockage of autophagy enhances anticancer efficacy of milatuzumab in mantle cell lymphoma: is FTY720 the next autophagy-blocking agent in lymphoma treatment? Autophagy 8:416–417. doi:10.4161/auto.19050
Alinari L, Mahoney E, Patton J, Zhang X, Huynh L, Earl CT, Mani R, Mao Y, Yu B, Quinion C, Towns WH, Chen CS, Goldenberg DM, Blum KA, Byrd JC, Muthusamy N, Praetorius-Ibba M, Baiocchi RA (2011) FTY720 increases CD74 expression and sensitizes mantle cell lymphoma cells to milatuzumab-mediated cell death. Blood 118:6893–6903. doi:10.1182/blood-2011-06-363879
Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ (2000) High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192:565–570. doi:10.1084/jem.192.4.565
Baetta R, Granata A, Canavesi M, Ferri N, Arnaboldi L, Bellosta S, Pfister P, Corsini A (2009) Everolimus inhibits monocyte/macrophage migration in vitro and their accumulation in carotid lesions of cholesterol-fed rabbits. J Pharmacol Exp Ther 328:419–425. doi:10.1124/jpet.108.144147
Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S (2008) mTOR pathway and mTOR inhibitors as agents for cancer therapy. Curr Cancer Drug Targets 8:647–665
Basso MD, Nambi P, Adelman SJ (2003) Effect of sirolimus on the cholesterol content of aortic arch in ApoE knockout mice. Transplant Proc 35:3136–3138. doi:10.1016/j.transproceed.2003.10.050
Bjornheden T, Bondjers G (1987) Oxygen consumption in aortic tissue from rabbits with diet-induced atherosclerosis. Arteriosclerosis 7:238–247
Clarke PG, Puyal J (2012) Autophagic cell death exists. Autophagy 8:867–869. doi:10.4161/auto.20380
Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GRY (2007) Selective clearance of macrophages in atherosclerotic plaques by the protein synthesis inhibitor cycloheximide. J Pharmacol Exp Ther 320:986–993. doi:10.1124/jpet.106.113944
Croons V, Martinet W, Herman AG, Timmermans JP, De Meyer GRY (2009) The protein synthesis inhibitor anisomycin induces macrophage apoptosis in rabbit atherosclerotic plaques through p38 mitogen-activated protein kinase. J Pharmacol Exp Ther 329:856–864. doi:10.1124/jpet.108.149948
De Meyer I, Martinet W, Van Hove CE, Schrijvers DM, Hoymans VY, Van VL, Fransen P, Bult H, De Meyer GRY (2011) Inhibition of inositol monophosphatase by lithium chloride induces selective macrophage apoptosis in atherosclerotic plaques. Br J Pharmacol 162:1410–1423. doi:10.1111/j.1476-5381.2010.01152.x
De Meyer I, Martinet W, Schrijvers DM, Timmermans J-P, Bult H, De Meyer GRY (2012) Toll-like receptor 7 stimulation by imiquimod induces macrophage autophagy and inflammation in atherosclerotic plaques. Basic Res Cardiol 107:269. doi:10.1007/s00395-012-0269-1
Decuypere JP, Bultynck G, Parys JB (2011) A dual role for Ca(2+) in autophagy regulation. Cell Calcium 50:242–250. doi:10.1016/j.ceca.2011.04.001
Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V (2008) Toll-like receptors control autophagy. EMBO J 27:1110–1121. doi:10.1038/emboj.2008.31
Denton D, Nicolson S, Kumar S (2012) Cell death by autophagy: facts and apparent artefacts. Cell Death Differ 19:87–95. doi:10.1038/cdd.2011.146
Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F (2009) Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11:1305–1314. doi:10.1038/ncb1975
Feingold KR, Kazemi MR, Magra AL, McDonald CM, Chui LG, Shigenaga JK, Patzek SM, Chan ZW, Londos C, Grunfeld C (2010) ADRP/ADFP and Mal1 expression are increased in macrophages treated with TLR agonists. Atherosclerosis 209:81–88. doi:10.1016/j.atherosclerosis.2009.08.042
Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF (2003) Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101:2652–2660. doi:10.1182/blood-2002-05-1300
Giordano A, Romano A (2011) Inhibition of human in-stent restenosis: a molecular view. Curr Opin Pharmacol 11:372–377. doi:10.1016/j.coph.2011.03.006
Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A (2008) Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J 410:525–534
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell 25:193–205. doi:10.1016/j.molcel.2006.12.009
Huang J, Lam GY, Brumell JH (2011) Autophagy signaling through reactive oxygen species. Antioxid Redox Signal 14:2215–2231. doi:10.1089/ars.2010.3554
Jia L, Hui RT (2009) Everolimus, a promising medical therapy for coronary heart disease? Med Hypotheses 73:153–155. doi:10.1016/j.mehy.2009.03.011
Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) mTOR regulation of autophagy. FEBS Lett 584:1287–1295. doi:10.1016/j.febslet.2010.01.017
Kalinina N, Agrotis A, Antropova Y, DiVitto G, Kanellakis P, Kostolias G, Ilyinskaya O, Tararak E, Bobik A (2004) Increased expression of the DNA-binding cytokine HMGB1 in human atherosclerotic lesions: role of activated macrophages and cytokines. Arterioscler Thromb Vasc Biol 24:2320–2325. doi:10.1161/01.ATV.0000145573.36113.8a
Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, White E (2007) Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev 21:1621–1635. doi:10.1101/quad.1565707
Keul P, Lucke S, von Wnuck LK, Bode C, Graler M, Heusch G, Levkau B (2011) Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res 108:314–323. doi:10.1161/CIRCRESAHA.110.235028
Keul P, Tolle M, Lucke S, von Wnuck LK, Heusch G, Schuchardt M, van der Giet M, Levkau B (2007) The sphingosine-1-phosphate analogue FTY720 reduces atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 27:607–613. doi:10.1161/01.ATV.0000254679.42583.88
Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, Ahn HJ, Ait-Mohamed O, Ait-Si-Ali S, Akematsu T, Akira S, Al-Younes HM, Al-Zeer MA, Albert ML, Albin RL, Alegre-Abarrategui J, Aleo MF, Alirezaei M, Almasan A, Almonte-Becerril M, Amano A, Amaravadi R, Amarnath S, Amer AO, Andrieu-Abadie N, Anantharam V, Ann DK, Anoopkumar-Dukie S, Aoki H, Apostolova N, Arancia G, Aris JP, Asanuma K, Asare NY, Ashida H, Askanas V, Askew DS, Auberger P, Baba M, Backues SK, Baehrecke EH, Bahr BA, Bai XY, Bailly Y, Baiocchi R, Baldini G, Balduini W, Ballabio A, Bamber BA, Bampton ET, Banhegyi G, Bartholomew CR, Bassham DC, Bast RC, Jr., Batoko H, Bay BH, Beau I, Bechet DM, Begley TJ, Behl C, Behrends C, Bekri S, Bellaire B, Bendall LJ, Benetti L, Berliocchi L, Bernardi H, Bernassola F, Besteiro S, Bhatia-Kissova I, Bi X, Biard-Piechaczyk M, Blum JS, Boise LH, Bonaldo P, Boone DL, Bornhauser BC, Bortoluci KR, Bossis I, Bost F, Bourquin JP, Boya P, Boyer-Guittaut M, Bozhkov PV, Brady NR, Brancolini C, Brech A, Brenman JE, Brennand A, Bresnick EH, Brest P, Bridges D, Bristol ML, Brookes PS, Brown EJ, Brumell JH, Brunetti-Pierri N, Brunk UT, Bulman DE, Bultman SJ, Bultynck G, Burbulla LF, Bursch W, Butchar JP, Buzgariu W, Bydlowski SP, Cadwell K, Cahova M, Cai D, Cai J, Cai Q, Calabretta B, Calvo-Garrido J, Camougrand N, Campanella M, Campos-Salinas J, Candi E, Cao L, Caplan AB, Carding SR, Cardoso SM, Carew JS, Carlin CR, Carmignac V, Carneiro LA, Carra S, Caruso RA, Casari G, Casas C, Castino R, Cebollero E, Cecconi F, Celli J, Chaachouay H, Chae HJ, Chai CY, Chan DC, Chan EY, Chang RC, Che CM, Chen CC, Chen GC, Chen GQ, Chen M, Chen Q, Chen SS, Chen W, Chen X, Chen X, Chen X, Chen YG, Chen Y, Chen Y, Chen YJ, Chen Z, Cheng A, Cheng CH, Cheng Y, Cheong H, Cheong JH, Cherry S, Chess-Williams R, Cheung ZH, Chevet E, Chiang HL, Chiarelli R, Chiba T, Chin LS, Chiou SH, Chisari FV, Cho CH, Cho DH, Choi AM, Choi D, Choi KS, Choi ME, Chouaib S, Choubey D, Choubey V, Chu CT, Chuang TH, Chueh SH, Chun T, Chwae YJ, Chye ML, Ciarcia R, Ciriolo MR, Clague MJ, Clark RS, Clarke PG, Clarke R, Codogno P, Coller HA, Colombo MI, Comincini S, Condello M, Condorelli F, Cookson MR, Coombs GH, Coppens I, Corbalan R, Cossart P, Costelli P, Costes S, Coto-Montes A, Couve E, Coxon FP, Cregg JM, Crespo JL, Cronje MJ, Cuervo AM, Cullen JJ, Czaja MJ, D’Amelio M, Darfeuille-Michaud A, Davids LM, Davies FE, De FM, de Groot JF, de Haan CA, De ML, De MA, De T, V, Debnath J, Degterev A, Dehay B, Delbridge LM, Demarchi F, Deng YZ, Dengjel J, Dent P, Denton D, Deretic V, Desai SD, Devenish RJ, Di GM, Di PG, Di PC, Diaz-Araya G, Diaz-Laviada I, Diaz-Meco MT, Diaz-Nido J, Dikic I, Dinesh-Kumar SP, Ding WX, Distelhorst CW, Diwan A, Djavaheri-Mergny M, Dokudovskaya S, Dong Z, Dorsey FC et al. (2012) Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 8:445–544. doi:10.4161/auto.19496
Larigauderie G, Furman C, Jaye M, Lasselin C, Copin C, Fruchart JC, Castro G, Rouis M (2004) Adipophilin enhances lipid accumulation and prevents lipid efflux from THP-1 macrophages: potential role in atherogenesis. Arterioscler Thromb Vasc Biol 24:504–510. doi:10.1161/01.ATV.0000115638.27381.97
Lavigne MC, Grimsby JL, Eppihimer MJ (2012) Antirestenotic mechanisms of everolimus on human coronary artery smooth muscle cells: inhibition of human coronary artery smooth muscle cell proliferation, but not migration. J Cardiovasc Pharmacol 59:165–174. doi:10.1097/FJC.0b013e31823a39c7
Law BY, Wang M, Ma DL, Al-Mousa F, Michelangeli F, Cheng SH, Ng MH, To KF, Mok AY, Ko RY, Lam SK, Chen F, Che CM, Chiu P, Ko BC (2010) Alisol B, a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca(2+) ATPase pump, induces autophagy, endoplasmic reticulum stress, and apoptosis. Mol Cancer Ther 9:718–730. doi:10.1158/1535-7163.MCT-09-0700
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688. doi:10.1172/JCI26390
Liao A, Hu R, Zhao Q, Li J, Li Y, Yao K, Zhang R, Wang H, Yang W, Liu Z (2012) Autophagy induced by FTY720 promotes apoptosis in U266 cells. Eur J Pharm Sci 45:600–605. doi:10.1016/j.ejps.2011.12.014
Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I (2012) Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab 15:545–553. doi:10.1016/j.cmet.2012.01.022
Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ (1999) Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 41:473–479
Ma KL, Ruan XZ, Powis SH, Moorhead JF, Varghese Z (2007) Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 292:H2721–H2728. doi:10.1152/ajpheart.01174.2006
Ma KL, Varghese Z, Ku Y, Powis SH, Chen Y, Moorhead JF, Ruan XZ (2010) Sirolimus inhibits endogenous cholesterol synthesis induced by inflammatory stress in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 298:H1646–H1651. doi:10.1152/ajpheart.00492.2009
Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ (2004) The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol 286:C507–C517. doi:10.1152/ajpcell.00201.2003
Martinet W, Agostinis P, Vanhoecke B, Dewaele M, De Meyer GRY (2009) Autophagy in disease: a double-edged sword with therapeutic potential. Clin Sci (Lond) 116:697–712. doi:10.1042/CS20080508
Martinet W, De Meyer GR (2008) Autophagy in atherosclerosis. Curr Atheroscler Rep 10:216–223
Martinet W, De Meyer GRY (2009) Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res 104:304–317. doi:10.1161/CIRCRESAHA.108.188318
Martinet W, De Meyer GRY, Andries L, Herman AG, Kockx MM (2006) Detection of autophagy in tissue by standard immunohistochemistry: possibilities and limitations. Autophagy 2:55–57
Martinet W, De Meyer GRY, Andries L, Herman AG, Kockx MM (2006) In situ detection of starvation-induced autophagy. J Histochem Cytochem 54:85–96. doi:10.1369/jhc.5A6743.2005
Martinet W, De Meyer I, Cools N, Timmerman V, Bult H, Bosmans J, De Meyer GRY (2010) Cell death-mediated cleavage of the attraction signal p43 in human atherosclerosis: implications for plaque destabilization. Arterioscler Thromb Vasc Biol 30:1415–1422. doi:10.1161/ATVBAHA.110.206029
Martinet W, De BM, Schrijvers DM, De Meyer GR, Herman AG, Kockx MM (2004) 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24:2296–2301. doi:10.1161/01.ATV.0000146266.65820.a1
Martinet W, Knaapen MW, De Meyer GRY, Herman AG, Kockx MM (2002) Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106:927–932. doi:10.1161/01.CIR.0000026393.47805.21
Martinet W, Schrijvers DM, De Meyer GRY (2012) Molecular and cellular mechanisms of macrophage survival in atherosclerosis. Basic Res Cardiol 107:297. doi:10.1007/s00395-012-0297-x
Martinet W, Verheye S, De Meyer GRY (2007) Everolimus-induced mTOR inhibition selectively depletes macrophages in atherosclerotic plaques by autophagy. Autophagy 3:241–244
Martinet W, Verheye S, De Meyer GRY (2007) Selective depletion of macrophages in atherosclerotic plaques via macrophage-specific initiation of cell death. Trends Cardiovasc Med 17:69–75. doi:10.1016/j.tcm.2006.12.004
Martinet W, Verheye S, De Meyer I, Timmermans JP, Schrijvers DM, Van Brussel I, Bult H, De Meyer GRY (2012) Everolimus triggers cytokine release by macrophages: rationale for stents eluting everolimus and a glucocorticoid. Arterioscler Thromb Vasc Biol 32:1228–1235. doi:10.1161/ATVBAHA.112.245381
Mathis AS, Jin S, Friedman GS, Peng F, Carl SM, Knipp GT (2007) The pharmacodynamic effects of sirolimus and sirolimus-calcineurin inhibitor combinations on macrophage scavenger and nuclear hormone receptors. J Pharm Sci 96:209–222. doi:10.1002/jps.20751
Matsuzawa T, Kim BH, Shenoy AR, Kamitani S, Miyake M, Macmicking JD (2012) IFN-gamma elicits macrophage autophagy via the p38 MAPK signaling pathway. J Immunol 189:813–818. doi:10.4049/jimmunol.1102041
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741. doi:10.1016/j.cell.2011.10.026
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. doi:10.1038/nature06639
Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J (2008) Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR−/− mice despite severe hypercholesterolemia. Atherosclerosis 198:39–48. doi:10.1016/j.atherosclerosis.2007.09.019
Muller C, Salvayre R, Negre-Salvayre A, Vindis C (2011) HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs. Cell Death Differ 18:817–828. doi:10.1038/cdd.2010.149
Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, Garcia-Garcia HM, Regar E, Kamberi M, Powers JC, Rapoza R, van BH, van der Giessen W, Virmani R (2010) Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 122:2288–2300. doi: 10.1161/CIRCULATIONAHA.109.921528
Ouimet M, Franklin V, Mak E, Liao X, Tabas I, Marcel YL (2011) Autophagy regulates cholesterol efflux from macrophage foam cells via lysosomal acid lipase. Cell Metab 13:655–667. doi:10.1016/j.cmet.2011.03.023
Petrovski G, Ayna G, Majai G, Hodrea J, Benko S, Madi A, Fesus L (2011) Phagocytosis of cells dying through autophagy induces inflammasome activation and IL-1beta release in human macrophages. Autophagy 7:321–330. doi:10.4161/auto.7.3.14583
Petrovski G, Zahuczky G, Majai G, Fesus L (2007) Phagocytosis of cells dying through autophagy evokes a pro-inflammatory response in macrophages. Autophagy 3:509–511
Razani B, Feng C, Coleman T, Emanuel R, Wen H, Hwang S, Ting JP, Virgin HW, Kastan MB, Semenkovich CF (2012) Autophagy links inflammasomes to atherosclerotic progression. Cell Metab 15:534–544. doi:10.1016/j.cmet.2012.02.011
Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC (2007) Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282:5641–5652. doi:10.1074/jbc.M609532200
Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111. doi:10.1083/jcb.200504035
Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O’Kane CJ, Schreiber SL, Rubinsztein DC (2007) Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol 3:331–338. doi:10.1038/nchembio883
Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC (2009) Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 16:46–56. doi:10.1038/cdd.2008.110
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195. doi:10.1038/nature00858
Schrijvers DM, De Meyer GRY, Kockx MM, Herman AG, Martinet W (2005) Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25:1256–1261. doi:10.1161/01.ATV.0000166517.18801.a7
Schrijvers DM, De Meyer GRY, Martinet W (2011) Autophagy in atherosclerosis: a potential drug target for plaque stabilization. Arterioscler Thromb Vasc Biol 31:2787–2791. doi:10.1161/ATVBAHA.111.224899
Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L, Dudek D (2009) A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373:897–910. doi:10.1016/S0140-6736(09)60325-1
Shalak V, Guigou L, Kaminska M, Wautier MP, Wautier JL, Mirande M (2007) Characterization of p43(ARF), a derivative of the p43 component of multiaminoacyl-tRNA synthetase complex released during apoptosis. J Biol Chem 282:10935–10943. doi:10.1074/jbc.M611737200
Shen S, Kepp O, Kroemer G (2012) The end of autophagic cell death? Autophagy 8:1–3. doi:10.4161/auto.8.1.16618
Shi CS, Kehrl JH (2008) MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem 283:33175–33182. doi:10.1074/jbc.M804478200
Sridhar S, Botbol Y, Macian F, Cuervo AM (2012) Autophagy and disease: always two sides to a problem. J Pathol 226:255–273. doi:10.1002/path.3025
Verheye S, Martinet W, Kockx MM, Knaapen MW, Salu K, Timmermans JP, Ellis JT, Kilpatrick DL, De Meyer GRY (2007) Selective clearance of macrophages in atherosclerotic plaques by autophagy. J Am Coll Cardiol 49:706–715. doi:10.1016/j.jacc.2006.09.047
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P (2010) AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem 285:9100–9113. doi:10.1074/jbc.M109.060061
Wang SH, Shih YL, Ko WC, Wei YH, Shih CM (2008) Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cell Mol Life Sci 65:3640–3652. doi:10.1007/s00018-008-8383-9
Wang X, Proud CG (2011) mTORC1 signaling: what we still don’t know. J Mol Cell Biol 3:206–220. doi:10.1093/jmcb/mjq038
Yang H, Wang H, Czura CJ, Tracey KJ (2005) The cytokine activity of HMGB1. J Leukoc Biol 78:1–8. doi:10.1189/jlb.1104648
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12:814–822. doi:10.1038/ncb0910-814
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304. doi:10.1074/jbc.M607007200
Zhang N, Qi Y, Wadham C, Wang L, Warren A, Di W, Xia P (2010) FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: a protective role of autophagy. Autophagy 6:1157–1167. doi:10.4161/auto.6.8.13614
Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T, Xiao GD, Yang YP, Liu CF (2010) The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun 394:377–382. doi:10.1016/j.bbrc.2010.03.026
Acknowledgments
This work was supported by the Fund for Scientific Research (FWO)-Flanders (projects G.0431.11N, G.0448.11N, G.0443.12N and G.0074.12N) and the University of Antwerp. The authors are indebted to Lieve Svensson, Francis Terloo and Dominique De Rijck for excellent technical assistance during transmission electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martinet, W., De Meyer, I., Verheye, S. et al. Drug-induced macrophage autophagy in atherosclerosis: for better or worse?. Basic Res Cardiol 108, 321 (2013). https://doi.org/10.1007/s00395-012-0321-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0321-1