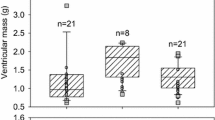
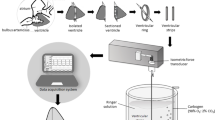
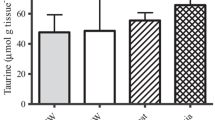
Abstract
This study investigated the effects of acclimation temperature upon (i) contractility of ventricular strips (ii) calcium movements in ventricular cardiomyocytes during excitation–contraction coupling (ECC), and (iii) the role of the sarcoplasmic reticulum (SR) in myocardial responses, in two marine teleosts, the sea bass (Dicentrarchus labrax) and the common sole (Solea solea). Because of the different sensitivities of their metabolism to temperature variation, both species were exposed to different thermal ranges. Sea bass were acclimated to 10, 15, 20, and 25 °C, and common sole to 6, 12, 18, and 24 °C, for 1 month. Isometric tension developed by ventricular strips was recorded over a range of physiological stimulation frequencies, whereas the depolarization-induced calcium transients were recorded on isolated ventricular cells through hyperpotassic solution application (at 100 mM). The SR contribution was assessed by ryanodine (RYAN) perfusion on ventricular strips and by caffeine application (at 10 mM) on isolated ventricular cells. Rates of contraction and relaxation of ventricular strip, in both species, increased with increasing acclimation temperature. At a low range of stimulation frequency, ventricular strips of common sole developed a positive force–frequency relationship at high acclimation temperature. In both the species, SR Ca2+-cycling was dependent on fish species, acclimation temperature and pacing frequency. The SR contribution was more important to force development at low acclimation temperatures in sea bass but at high acclimation temperatures in common sole. The results also revealed that high acclimation temperature causes an increase in the maximum calcium response amplitude on ventricular cells in both the species. Although sea bass and common sole occupy similar environments and tolerate similar environmental temperatures, this study indicated that sea bass and common sole can acclimatize to new thermal conditions, adjusting their cellular process in a different manner.







Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- CICR:
-
Ca2+-induced Ca2+-release
- DMSO:
-
Dimethyl sulfoxide
- ECC:
-
Excitation-contraction coupling
- EGTA:
-
Ethylene glycol tetraacetic acid
- ICaL:
-
L-type Ca2+-current
- NCX:
-
Na+/Ca2+exchanger
- PMCA:
-
Sarcolemmal Ca2+-ATPase pump
- RYAN:
-
Ryanodine
- SERCA:
-
SR Ca2+-ATPase pumps
- SL:
-
Sarcolemma
- SR:
-
Sarcoplasmic reticulum
References
Aho E, Vornanen M (1998) Ca2+ -ATPase activity and Ca2+ uptake by the sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J Exp Biol 201:525–532
Aho E, Vornanen M (1999) Contractile properties of atrial and ventricular tissue of the rainbow trout (Oncorhynchus mykiss) heart: effects of thermal acclimation. J Exp Biol 202:2663–2677
Bassani JW, Weilong Y, Bers DM (1995) Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol 268:C1313–C1329
Birkedal R, Christopher J, Thistlethwaite A, Shiels H (2009) Temperature acclimation has no effect on ryanodine receptor expression or subcellular localization in rainbow trout heart. J Comp Physiol B 179:961–969
Bowler K, Tirri R (1990) Temperature dependence of the heart isolated from the cold or warm acclimated perch (Perca fluviatilis). Comp Biochem Physiol A 96:177–180
Castilho PC, Landeira-Fernandez AM, Block BA (2007) Elevated Ca2+ ATPase (SERCA2) activity in tuna hearts: comparative aspects of temperature dependence. Comp Biochem Physiol A 148:124–132
Chatelier A, Imbert N, Zambonino Infante JL, McKenzie DJ, Bois P (2006) Effects of oleic acid on the high threshold barium current in sea bass (Dicentrarchus labrax) ventricular myocytes. J Exp Biol 209:4033–4039
Claireaux G, Lagardere JP (1999) Influence of temperature, oxygen and salinity on the metabolism of European sea bass. J Sea Res 42:157–168
Cohen O, Kanana H, Zoizner R, Gross C, Meiri U, Stern M, Gerstenblith G, Horowitz M (2006) Altered Ca2_ handling and myofilament desensitization underlie cardiomyocyte performance in normothermic and hyperthermic heat-acclimated rat hearts. J Appl Physiol 103:266–275
Driedzic WR, Bailey JR, Septon DH (1996) Cardiac adaptations to low temperature in non-polar teleost fish. J Exp Zool 275:186–195
Erickson JR, Sidell BD, Moerland TM (2005) Temperature sensitivity of calcium binding for parvalbumins from Antarctic and temperate zone teleost fishes. Comp Biochem Physiol A 140:179–185
Farrell AP (1997) Effects of temperature on cardiovascular performance. In: Wood CM, MacDonald DG (eds) Global warming implications for freshwater and marine fish, society for experimental biology, seminar series 61. Cambridge University Press, Cambridge, pp 135–153
Galli GL, Lipnick MS, Block BA (2009) Effect of thermal acclimation on action potentials and sarcolemmal K+ channels from Pacific bluefin tuna cardiomyocytes. Am J Physiol 297(2):R502–509
Galli GL, Lipnick MS, Shiels HA, Block BA (2011) Temperature effects on Ca2 + cycling in scombrid cardiomyocytes: a phylogenetic comparison. J Exp Biol 214:1068–1076
Gamperl AK, Wilkinson M, Boutilier RG (1994) β-Adrenoreceptors in the trout (Oncorhynchus mykiss) heart: characterization, quantification and effects of repeated catecholamine exposure. Gen Comp Endocr 95:259–272
Gomez JP, Potreau D, Raymond G (1994) Intracellular calcium transients from newborn rat cardiomyocytes in primary culture. Cell Calcium 15:265–275
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved florescence properties. J Biol Chem 260:3440–3450
Györke S, Terentyev D (2008) Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res 77:245–255
Harwood CL, Howarth FC, Altringham JD, White ED (2000) Rate-dependant changes in cell shortening, intracellular Ca2+ levels and membrane potential in single, isolated rainbow trout (Onchorhynchus mykiss) ventricular myocytes. J Exp Biol 203:493–504
Haverinen J, Vornanen M (2009) Comparison of sarcoplasmic reticulum calcium content in atrial and ventricular myocytes of three fish species. Am J Physiol 297:R1180–R1187
Hove-Madsen L (1992) The influence of temperature on ryanodine sensitivity and the force–frequency relationship in the myocardium of rainbow trout. J Exp Biol 167:47–60
Hove-Madsen L, Llach A, Tort L (1998) Quantification of Ca2+ uptake in the sarcoplasmic reticulum of trout ventricular myocytes. Am J Physiol 275:R2070–R2080
Keen JE, Farrell AP, Tibbits GF, Brill RW (1992) Cardiac physiology in tunas. II. Effect of ryanodine, calcium, and adrenaline on force–frequency relationships in atrial strips from skipjack tuna, Katsuwonus Pelamis. Can J Zool 70:1211–1217
Keen JE, Viazon DM, Farrell AP, Tibbits GF (1994) Effect of temperature acclimation on the ryanodine sensitivity of the trout myocardium. J Comp Physiol B 164:438–443
Landeira-Fernandez AM, Morrissette JM, Blank JM, Block BA (2004) Temperature dependence of the Ca2+-ATPase (SERCA2) in the ventricles of tuna and mackerel. Am J Physiol 286:R398–R404
Larsson D, Larsson B, Lundgren T, Sundell K (1999) The effect of pH and temperature on the dissociation constant for Fura-2 and their effects on [Ca2+]i in enterocytes from a poikilothermic animal, Atlantic cod (Gadus morhua). Anat Biochem 273:60–65
Lefrançois C, Claireaux G (2003) Influence of ambient oxygenation and temperature on metabolic scope and heart rate of common sole (Solea solea). Mar Ecol Prog Ser 259:273–284
Matikainen N, Vornanen M (1992) Effect of season and temperature acclimation on the function of crucian carp (Carassius carassius) heart. J Exp Biol 167:203–220
Mercier C, Axelsson M, Imbert N, Claireaux G, Lefrançois C, Altimiras J, Farrell AP (2002) In vitro cardiac performance in triploid brown trout at two acclimation temperatures. J Fish Biol 50:117–133
Moller-Nielsen T, Gesser H (1992) Sarcoplasmic reticulum and excitation-contraction coupling at 10 and 20 °C in rainbow trout myocardium. J Comp Physiol B 162:526–534
Orchard CH, Lakatta EG (1985) Intracellular calcium transients and developed tension in rat heart muscle. J Gen Physiol 86:637–651
Pelouch V, Vornanen M (1996) Effects of thermal acclimation on ventricle size, protein composition, and contractile properties of crucian carp heart. J Therm Biol 2(I):1–9
Randall DJ, Perry SF (1992) Catecholamines. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology: the cardiovascular system. Academic Press, New York, pp 255–300
Rivaroli L, Rantin FT, Kalinin AL (2006) Cardiac function of two ecologically distinct Neotropical freshwater fish: Curimbata, Prochilodus lineatus (Teleostei, Prochilodontidae), and trahira, Hoplias malabaricus (Teleostei, Erythrinidae). Comp Biochem Physiol A 145(3):322–327
Rousseau E, Smith JS, Meissner G (1987) Ryanodine modifies conductance and gating behaviour of single Ca2+ release channels. Am J Physiol 253:C364–C368
Shattock MJ, Bers DM (1987) Inotropic response to hypothermia and the temperature-dependence of ryanodine action in isolated rabbit and rat ventricular muscle: implications for E-C coupling. Cir Res 61:761–771
Shiels HA, Farrell AP (1997) The effects of temperature and adrenaline on the relative importance of the sarcoplasmic reticulum in the contributing Ca2+ to force development in isolated ventricular trabeculae from rainbow trout. J Exp Biol 200:1607–1621
Shiels HA, Farrell AP (2000) The effect of ryanodine on isometric tension development in isolated ventricular trabeculae from Pacific mackerel (Scomber japonicus). Comp Biochem Physiol A 125:331–341
Shiels HA, Stevens ED, Farrell AP (1998) Effect of temperature, adrenaline and ryanodine on power production in trout (Oncorhynchus mykiss) ventricular trabeculae. J Exp Biol 201:2701–2710
Shiels HA, Freund EV, Farrell AP, Block BA (1999) The sarcoplasmic reticulum plays a major role in isometric contraction in atrial muscle of yellowfin tuna. J Exp Biol 202:881–890
Shiels HA, Vornanen M, Farrell AP (2002) The force–frequency relationship in fish heart. A review. Comp Biochem Physiol A 132:811–826
Tiitu V, Vornanen M (2001) Cold adaptation suppresses the contractility of both atrial and ventricular muscle of the crucian carp heart. J Fish Biol 59:141–156
Tiitu V, Vornanen M (2003) Ryanodine and dihydropyridine receptor binding in ventricular cardiac muscle of fish with different temperature preferences. J Comp Physiol B 173(4):285–291
Vornanen M (1989) Regulation of contractility of the fish (Carassius carassius L.) heart ventricle. Comp Biochem Physiol C 94:477–483
Vornanen M (1997) Sarcolemmal Ca influx through L-type Ca channels in the ventricular myocytes of a teleost fish. Am J Physiol 272:1432–1440
Vornanen M (1998) L-type Ca2+ current in fish cardiac myocytes: effects of thermal acclimation and β-adrenergic stimulation. J Exp Biol 201:533–547
Vornanen M (1999) Na+/Ca2+ exchange current in ventricular myocytes of fish heart: contribution to sarcolemmal Ca2+ influx. J Exp Biol 202:1763–1775
Vornanen M (2006) Temperature and Ca2+- dependence of [3H]ryanodine binding in the burbot (Lota lota L.) heart. Am J Physiol 290:R345–R351
Vornanen M, Shiels HA, Farrell AP (2002a) Plasticity of excitation-contraction coupling in fish cardiac myocytes. Comp Biochem Physiol A 132(4):827–846
Vornanen M, Ryökkynen A, Nurmi A (2002b) Temperature-dependent expression of sarcolemmal K+ currents in rainbow trout atrial and ventricular myocytes. Am J Physiol 282:R1191–R1199
Yue DT (1992) Relationships between intracellular free calcium and force with changes of interval. In: Noble MI, Seed WA (eds) The interval-force relationship of the heart: bowditch revisited. Cambridge University Press., Cambridge, pp 95–109
Acknowledgments
We are indebted to the Aquarium of La Rochelle and N.Vallee for their technical assistance. The study was funded by the Région Poitou–Charentes, the CNRS and IFREMER.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Imbert-Auvray, N., Mercier, C., Huet, V. et al. Sarcoplasmic reticulum: a key factor in cardiac contractility of sea bass Dicentrarchus labrax and common sole Solea solea during thermal acclimations. J Comp Physiol B 183, 477–489 (2013). https://doi.org/10.1007/s00360-012-0733-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0733-0