Abstract
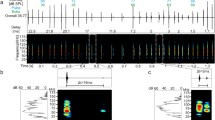
Previous studies in echolocating bats, Myotis lucifugus, showed that paradoxical latency shift (PLS) is essential for neural computation of target range and that a number of neurons in the inferior colliculus (IC) exhibit unit-specific PLS (characterized by longer first-spike latency at higher sound levels) in response to tone pulses at the unit’s best frequency. The present study investigated whether or not frequency-modulated (FM) pulses that mimic the bat’s echolocation sonar signals were equally effective in eliciting PLS. For two-thirds of PLS neurons in the IC, both FM and tone pulses could elicit PLS, but only FM pulses consistently produced unit-specific PLS. For the remainder of PLS neurons, only FM pulses effectively elicited PLS; these cells showed either no PLS or no response, to tone pulses. PLS neurons generally showed more pronounced PLS in response to narrow-band FM (each sweeping 20 kHz in 2 ms) pulse that contained the unit’s best frequency. In addition, almost all PLS neurons showed duration-independent PLS to FM pulses, but the same units exhibited duration-dependent PLS to tone pulses. Taken together, when compared to tone pulses, FM stimuli can provide more reliable estimates of target range.




Similar content being viewed by others
References
Berkowitz A, Suga N (1989) Neural mechanisms of ranging are different in two species of bats. Hear Res 41:255–264
Bodenhamer RD, Pollak GD (1981) Time and frequency domain processing in the inferior colliculus of echolocating bats. Hear Res 5:317–335
Casseday JH, Covey E (1992) Frequency tuning properties of neurons in the inferior colliculus of an FM bat. J Comp Neurol 319:34–50
Casseday JH, Ehrlich D, Covey E (1994) Neuronal tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science 264:847–850
Casseday JH, Covey E, Grothe B (1997) Neural selectivity and tuning for sinusoidal frequency modulations in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol 77:1595–1605
Covey E (1993) Response properties of single units in the dorsal nucleus of the lateral lemniscus and paralemniscal zone of an echolocating bat. J Neurophysiol 69:842–859
Ehrlich D, Casseday JH, Covey E (1997) Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol 77:2360–2370
Feng AS, Simmons JA, Kick SA (1978) Echo detection and target-ranging neurons in the auditory system of the bat Eptesicus fuscus. Science 202:645–648
Fitzpatrick DC, Suga N, Misawa H (1991) Are the initial frequency-modulated components of the mustached bat’s biosonar pulses important for ranging? J Neurophysiol 66:951–964
Fuzessery ZM (1994) Response selectivity for multiple dimensions of frequency sweeps in the pallid bat inferior colliculus. J Neurophysiol 72(3):1061–1079
Fuzessery ZM, Hall JC (1996) Role of GABA in shaping frequency tuning and creating FM sweep selectivity in the inferior colliculus. J Neurophysiol 76:1059–1073
Galazyuk AV, Feng AS (2001) Oscillation may play a role in time domain central auditory processing. J Neurosci 21(RC147):1–5
Galazyuk AV, Lin W, Llano D, Feng AS (2005) Leading inhibition to neural oscillation is important for time-domain processing in the auditory midbrain. J Neurophysiol 94:314–326
Ghose K, Moss CF (2003) The sonar beam pattern of a flying bat as it tracks tethered insects. J Acoust Soc Am 114:1120–1131
Gordon M, O’Neill WE (2000) An extralemniscal component of the mustached bat inferior colliculus selective for direction and rate of linear frequency modulations. J Comp Neurol 426:165–181
Klug A, Khan A, Burger RM, Bauer EE, Hurley LM, Yang L, Grothe B, Halvorsen MB, Park TJ (2000) Latency as a function of intensity in auditory neurons: influences of central processing. Hear Res 148:107–123
Langner G (1992) Periodicity coding in the auditory system. Hear Res 60:115–142
Langner G, Schreiner CE (1988) Periodicity coding in the inferior colliculus of the cat. I. Neuronal mechanisms. J Neurophysiol 60:1799–1822
Maekawa M, Wong D, Paschal WG (1992) Spectral selectivity of FM–FM neurons in the auditory cortex of the echolocating bat, Myotis lucifugus. J Comp Physiol A171:513–522
Mittmann DH, Wenstrup JJ (1995) Combination-sensitive neurons in the inferior colliculus. Hear Res 90:185–191
Neuweiler G (1984) Foraging, echolocation and audition in bats. Naturwissenschaften 71:446–455
O’Neill WE (1995) The bat auditory cortex. In: Popper AN, Fay RR (eds) Hearing by bats. Springer, Berlin Heidelberg New York, pp 416–498
O’Neill WE, Brimijoin WO (2002) Directional selectivity for FM sweeps in the suprageniculate nucleus of the mustached bat medial geniculate body. J Neurophysiol 88:172–187
O’Neill WE, Suga N (1979) Target range-sensitive neurons in the auditory cortex of the mustache bat. Science 203:69–73
Olsen JF, Suga N (1991a) Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of target range information. J Neurophysiol 65:1275–1296
Olsen JF, Suga N (1991b) Combination-sensitive neurons in the medial geniculate body of the mustached bat: encoding of relative velocity information. J Neurophysiol 65:1254–1274
Pinheiro AD, Wu M, Jen PH (1991) Encoding repetition rate and duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Comp Physiol A169:69–85
Poon PW, Chen X, Cheung YM (1992) Differences in FM response correlate with morphology of neurons in the rat inferior colliculus. Exp Brain Res 91:94–104
Rees A, Moller AR (1983) Responses of neurons in the inferior colliculus of the rat to AM and FM tones. Hear Res 10:301–330
Roverud RC (1993) Neural computations for sound pattern recognition: evidence for summation of an array of frequency filters in an echolocating bat. J Neurosci 13:2306–2312
Sanderson MI, Simmons JA (2005) Target representation of naturalistic echolocation sequences in single unit responses from the inferior colliculus of big brown bats. J Acoust Soc Am 118:3352–3361
Schuller G (1979) Coding of small sinusoidal frequency and amplitude modulations in the inferior colliculus of ‘CF–FM’ bat, Rhinolophus ferrumequinum. Exp Brain Res 34:117–132
Shannon-Hartman S, Wong D, Maekawa M (1992) Processing of pure-tone and FM stimuli in the auditory cortex of the FM bat, Myotis lucifugus. Hear Res 61:179–188
Simmons JA (1973) The resolution of target range by echolocating bats. J Acoust Soc Am 54:157–173
Simmons JA (1979) Perception of echo phase information in bat sonar. Science 204:1336–1338
Suga N (1965) Analysis of frequency modulated sounds by neurons of echolocating bats. J Physiol 179:26–53
Suga N (1968) Analysis of frequency modulated and complex sounds by single auditory neurons of bats. J Physiol 198:51–80
Suga N (1969) Classification of inferior collicular neurons of bats in terms of responses to pure tones, FM sounds and noise bursts. J Physiol 200:555–574
Suga N, O’Neill WE, Kujirai K, Manabe T (1983) Specificity of combination-sensitive neurons for processing of complex biosonar signals in auditory cortex of the mustached bat. J Neurophysiol 49:1573–1626
Sullivan WE (1982a) Neural representation of target distance in auditory cortex of the echolocating bat Myotis lucifugus. J Neurophysiol 48:1011–1032
Sullivan WE (1982b) Possible neural mechanisms of target distance coding in auditory system of the echolocating bat Myotis lucifugus. J Neurophysiol 48:1033–1047
Surlykke A, Moss CF (2000) Echolocation behavior of big brown bats, Eptesicus fuscus, in the field and the laboratory. J Acoust Soc Am 108:2419–2429
Wadsworth J, Moss CF (2000) Vocal control of acoustic information for sonar discriminations by the echolocating bat, Eptesicus fuscus. J Acoust Soc Am 107:2265–2271
Acknowledgment
This research is supported by a grant from the National Institute on Deafness and Communication Disorders of the NIH (R01DC04998). We thank Wenyu Lin and Karla Melendez for their comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Galazyuk, A.V. & Feng, A.S. FM signals produce robust paradoxical latency shifts in the bat’s inferior colliculus. J Comp Physiol A 193, 13–20 (2007). https://doi.org/10.1007/s00359-006-0167-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-006-0167-9