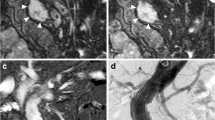
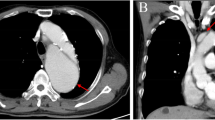
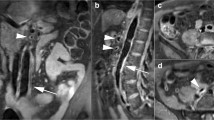
Abstract
Objectives
Management of abdominal aortic aneurysms (AAAs) is based on diameter. CT angiography (CTA) is commonly used, but requires radiation and iodinated contrast. Non-contrast MRI is an appealing alternative that may allow better characterization of intraluminal thrombus (ILT). This study aims to 1) validate non-contrast MRI for measuring AAA diameter, and 2) to assess ILT with CTA and MRI.
Method
28 patients with AAAs (diameter 50.7 ± 12.3 mm) underwent CTA and non-contrast MRI. MRI was acquired at 3 T using 1) a conventional 3D gradient echo (GRE) sequence and 2) a 3D T1-weighted black blood fast-spin-echo sequence. Two radiologists independently measured the AAA diameter. The ratio of signal of ILT and adjacent psoas muscle (ILTr = signalILT/signalMuscle) was quantified.
Results
Strong agreement between CTA and non-contrast MRI was shown for AAA diameter (intra-class coefficient > 0.99). Both approaches had excellent inter-observer reproducibility (ICC > 0.99). ILT appeared homogenous on CTA, whereas MRI revealed compositional variations. Patients with AAAs ≥5.5 cm and <5.5 cm had a variety of distributions of old/fresh ILT types.
Conclusions
Non-contrast 3D black blood MRI provides accurate and reproducible AAA diameter measurements as validated by CTA. It also provides unique information about ILT composition, which may be linked with elevated risk for disease progression.
Key Points
• Non-contrast MRI is an appealing alternative to CTA for AAA management.
• Non-contrast MRI can accurately measure AAA diameters compared to CTA.
• MRI affords unique characterization of intraluminal thrombus composition.





Similar content being viewed by others
Abbreviations
- AAA:
-
Abdominal aortic aneurysm
- CTA:
-
Computed tomography angiography
- ILT:
-
Intra-luminal thrombus
- GRE:
-
Gradient echo
- FOV:
-
Field of view
- MPR:
-
Multi-planar reconstruction
- ICC:
-
Intra-class correlation coefficient
- CV:
-
Coefficient of variance
- LOA:
-
Limits of agreement
- FSE:
-
Fast spin echo.
References
Kent KC, Zwolak RM, Egorova NN et al (2010) Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 52:539–548
Derubertis BG, Trocciola SM, Ryer EJ et al (2007) Abdominal aortic aneurysm in women: prevalence, risk factors, and implications for screening. J Vasc Surg 46:630–635
LeFevre ML (2014) Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 161:281–290
Beales L, Wolstenhulme S, Evans JA, West R, Scott DJ (2011) Reproducibility of ultrasound measurement of the abdominal aorta. Br J Surg 98:1517–1525
Nguyen VL, Leiner T, Hellenthal FA et al (2014) Abdominal aortic aneurysms with high thrombus signal intensity on magnetic resonance imaging are associated with high growth rate. Eur J Vasc Endovasc Surg 48:676–684
Behr-Rasmussen C, Grondal N, Bramsen MB, Thomsen MD, Lindholt JS (2014) Mural thrombus and the progression of abdominal aortic aneurysms: a large population-based prospective cohort study. Eur J Vasc Endovasc Surg 48:301–307
Stenbaek J, Kalin B, Swedenborg J (2000) Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 20:466–469
Wolf YG, Thomas WS, Brennan FJ, Goff WG, Sise MJ, Bernstein EF (1994) Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. J Vasc Surg 20:529–535, discussion 535–528
Speelman L, Schurink GW, Bosboom EM et al (2010) The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J Vasc Surg 51:19–26
Metaxa E, Kontopodis N, Tzirakis K, Ioannou CV, Papaharilaou Y (2015) Effect of intraluminal thrombus asymmetrical deposition on abdominal aortic aneurysm growth rate. J Endovasc Ther 22:406–412
Vorp DA, Lee PC, Wang DH et al (2001) Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg 34:291–299
Kazi M, Thyberg J, Religa P et al (2003) Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg 38:1283–1292
Martinez-Pinna R, Madrigal-Matute J, Tarin C et al (2013) Proteomic analysis of intraluminal thrombus highlights complement activation in human abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 33:2013–2020
Nchimi A, Courtois A, El Hachemi M et al (2016) Multimodality imaging assessment of the deleterious role of the intraluminal thrombus on the growth of abdominal aortic aneurysm in a rat model. Eur Radiol 26:2378–2386
Richards JM, Semple SI, MacGillivray TJ et al (2011) Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ Cardiovasc Imaging 4:274–281
Veldhoen S, Behzadi C, Derlin T et al (2015) Exact monitoring of aortic diameters in Marfan patients without gadolinium contrast: intraindividual comparison of 2D SSFP imaging with 3D CE-MRA and echocardiography. Eur Radiol 25:872–882
Zhu C, Haraldsson H, Faraji F et al (2016) Isotropic 3D black blood MRI of abdominal aortic aneurysm wall and intraluminal thrombus. Magn Reson Imaging 34:18–25
Li L, Miller KL, Jezzard P (2012) DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med 68:1423–1438
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Brady AR, Thompson SG, Fowkes FG, Greenhalgh RM, Powell JT (2004) Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 110:16–21
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE (2007) Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242:647–649
Goshima S, Kanematsu M, Kondo H et al (2013) Preoperative planning for endovascular aortic repair of abdominal aortic aneurysms: feasibility of nonenhanced MR angiography versus contrast-enhanced CT angiography. Radiology 267:948–955
Guirguis-Blake JM, Beil TL, Senger CA, Whitlock EP (2014) Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 160:321–329
den Hartog AW, Franken R, de Witte P et al (2013) Aortic disease in patients with Marfan syndrome: aortic volume assessment for surveillance. Radiology 269:370–377
Hope MD, Hope TA, Zhu C et al (2015) Vascular imaging with ferumoxytol as a contrast agent. AJR Am J Roentgenol 205:W366–W373
Li ZY, Sadat U, J UK-I et al (2010) Association between aneurysm shoulder stress and abdominal aortic aneurysm expansion: a longitudinal follow-up study. Circulation 122:1815–1822
Roy J, Labruto F, Beckman MO, Danielson J, Johansson G, Swedenborg J (2008) Bleeding into the intraluminal thrombus in abdominal aortic aneurysms is associated with rupture. J Vasc Surg 48:1108–1113
Zhu C, Haraldsson H, Tian B et al (2016) High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI. MAGMA 29:559–570
Chu B, Kampschulte A, Ferguson MS et al (2004) Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35:1079–1084
Zhu C, Sadat U, Patterson AJ, Teng Z, Gillard JH, Graves MJ (2014) 3D high-resolution contrast enhanced MRI of carotid atheroma--a technical update. Magn Reson Imaging 32:594–597
Teng Z, Feng J, Zhang Y et al (2015) Layer- and direction-specific material properties, extreme extensibility and ultimate material strength of human abdominal aorta and aneurysm: a uniaxial extension study. Ann Biomed Eng 43:2745–2759
O'Leary SA, Kavanagh EG, Grace PA, McGloughlin TM, Doyle BJ (2014) The biaxial mechanical behaviour of abdominal aortic aneurysm intraluminal thrombus: classification of morphology and the determination of layer and region specific properties. J Biomech 47:1430–1437
Sadat U, Teng Z, Young VE et al (2011) Impact of plaque haemorrhage and its age on structural stresses in atherosclerotic plaques of patients with carotid artery disease: an MR imaging-based finite element simulation study. Int J Cardiovasc Imaging 27:397–402
Acknowledgments
The scientific guarantor of this publication is Michael D. Hope. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding by United States National Institutes of Health (NIH) grants R01HL114118 and R01HL123759. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study.
Methodology: retrospective, observational, multicenter study
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chengcheng Zhu and Bing Tian contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, C., Tian, B., Leach, J.R. et al. Non-contrast 3D black blood MRI for abdominal aortic aneurysm surveillance: comparison with CT angiography. Eur Radiol 27, 1787–1794 (2017). https://doi.org/10.1007/s00330-016-4559-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4559-0