Abstract
The innate immune system is essential for the detection and elimination of bacterial pathogens. Upon inflammasome activation, caspase-1 cleaves pro-IL-1β and pro-IL-18 to their mature forms IL-1β and IL-18, respectively, and the cell undergoes inflammatory death termed pyroptosis. Here, we reviewed recent findings demonstrating that Brucella abortus ligands activate NLRP3 and AIM2 inflammasomes which lead to control of infection. This protective effect is due to the inflammatory response caused by IL-1β and IL-18 rather than cell death. Brucella DNA is sensed by AIM2 and bacteria-induced mitochondrial reactive oxygen species is detected by NLRP3. However, deregulation of pro-inflammatory cytokine production can lead to immunopathology. Nervous system invasion by bacteria of the genus Brucella results in an inflammatory disorder termed neurobrucellosis. Herein, we discuss the mechanism of caspase-1 activation and IL-1β secretion in glial cells infected with B. abortus. Our results demonstrate that the ASC inflammasome is indispensable for inducing the activation of caspase-1 and secretion of IL-1β upon infection of astrocytes and microglia with Brucella. Moreover, our results demonstrate that secretion of IL-1β by Brucella-infected glial cells depends on NLRP3 and AIM2 and leads to neurobrucellosis. Further, the inhibition of the host cell inflammasome as an immune evasion strategy has been described for bacterial pathogens. We discuss here that the bacterial type IV secretion system VirB is required for inflammasome activation in host cells during infection. Taken together, our results indicate that Brucella is sensed by ASC inflammasomes mainly NLRP3 and AIM2 that collectively orchestrate a robust caspase-1 activation and pro-inflammatory response.


Similar content being viewed by others
References
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34(5):637–650. doi:10.1016/j.immuni.2011.05.006
Albiger B, Dahlberg S, Henriques-Normark B, Normark S (2007) Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med 261(6):511–528. doi:10.1111/j.1365-2796.2007.01821.x
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140(6):821–832. doi:10.1016/j.cell.2010.01.040
Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30(1):16–34. doi:10.3109/08830185.2010.529976
Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M Jr, Akira S, Yonehara S, Kato A, Fujita T (2005) Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 175(5):2851–2858
Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, Ohba Y, Taniguchi T (2007) DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448(7152):501–505. doi:10.1038/nature06013
Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA (2009) AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458(7237):514–518. doi:10.1038/nature07725
Sun L, Wu J, Du F, Chen X, Chen ZJ (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339(6121):786–791. doi:10.1126/science.1232458
Ishikawa H, Barber GN (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455(7213):674–678. doi:10.1038/nature07317
Iwasaki A, Medzhitov R (2004) Toll-like receptor control of the adaptive immune responses. Nat Immunol 5(10):987–995. doi:10.1038/ni1112
Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS (2012) Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11(5):469–480. doi:10.1016/j.chom.2012.03.007
Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol 11(5):395–402. doi:10.1038/ni.1864
Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray PJ, Muller M, Decker T (2004) IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J Immunol 173(12):7416–7425
Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM (2010) Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A 107(21):9771–9776. doi:10.1073/pnas.1003738107
Huang LY, Ishii KJ, Akira S, Aliberti J, Golding B (2005) Th1-like cytokine induction by heat-killed Brucella abortus is dependent on triggering of TLR9. J Immunol 175(6):3964–3970
Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC (2013) Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol 190(7):3629–3638. doi:10.4049/jimmunol.1202817
Corbel MJ (1997) Brucellosis: an overview. Emerg Infect Dis 3(2):213–221. doi:10.3201/eid0302.970219
Boschiroli ML, Foulongne V, O’Callaghan D (2001) Brucellosis: a worldwide zoonosis. Curr Opin Microbiol 4(1):58–64
Golding B, Scott DE, Scharf O, Huang LY, Zaitseva M, Lapham C, Eller N, Golding H (2001) Immunity and protection against Brucella abortus. Microbes Infect 3(1):43–48
Oliveira SC, Splitter GA (1995) CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur J Immunol 25(9):2551–2557. doi:10.1002/eji.1830250922
Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP (2003) Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J Exp Med 198(4):545–556. doi:10.1084/jem.20030088
Celli J, Tsolis RM (2015) Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes? Nat Rev Microbiol 13(2):71–82. doi:10.1038/nrmicro3393
Giambartolomei GH, Zwerdling A, Cassataro J, Bruno L, Fossati CA, Philipp MT (2004) Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J Immunol 173(7):4635–4642
Pasquevich KA, Garcia Samartino C, Coria LM, Estein SM, Zwerdling A, Ibanez AE, Barrionuevo P, Oliveira FS, Carvalho NB, Borkowski J, Oliveira SC, Warzecha H, Giambartolomei GH, Cassataro J (2010) The protein moiety of Brucella abortus outer membrane protein 16 is a new bacterial pathogen-associated molecular pattern that activates dendritic cells in vivo, induces a Th1 immune response, and is a promising self-adjuvanting vaccine against systemic and oral acquired brucellosis. J Immunol 184(9):5200–5212. doi:10.4049/jimmunol.0902209
Gomes MT, Campos PC, Pereira GS, Bartholomeu DC, Splitter G, Oliveira SC (2016) TLR9 is required for MAPK/NF-kappaB activation but does not cooperate with TLR2 or TLR6 to induce host resistance to Brucella abortus. J Leukoc Biol 99(5):771–780. doi:10.1189/jlb.4A0815-346R
Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC (2008) Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol 180(2):1080–1087
Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD (2015) The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol 16(5):467–475. doi:10.1038/ni.3118
Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10(3):241–247. doi:10.1038/ni.1703
Belhocine K, Monack DM (2012) Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells. Cell Microbiol 14(1):71–80. doi:10.1111/j.1462-5822.2011.01700.x
Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol 11(5):385–393. doi:10.1038/ni.1859
Storek KM, Monack DM (2015) Bacterial recognition pathways that lead to inflammasome activation. Immunol Rev 265(1):112–129. doi:10.1111/imr.12289
Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, Comerci DJ, Ugalde RA, Pierre P, Gorvel JP (2008) Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog 4(2):e21. doi:10.1371/journal.ppat.0040021
Shah S, Bohsali A, Ahlbrand SE, Srinivasan L, Rathinam VA, Vogel SN, Fitzgerald KA, Sutterwala FS, Briken V (2013) Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system. J Immunol 191(7):3514–3518. doi:10.4049/jimmunol.1301331
Pang Z, Sun G, Junkins RD, Lin TJ (2015) AIM2 inflammasome is dispensable for the host defense against Pseudomonas aeruginosa infection. Cell Mol Biol (Noisy-le-Grand) 61(3):63–70
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426
Aganna E, Martinon F, Hawkins PN, Ross JB, Swan DC, Booth DR, Lachmann HJ, Bybee A, Gaudet R, Woo P, Feighery C, Cotter FE, Thome M, Hitman GA, Tschopp J, McDermott MF (2002) Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum 46(9):2445–2452. doi:10.1002/art.10509
Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, Mak S, Lora JM, Briskin M, Jurman M, Cao J, DiStefano PS, Bertin J (2002) PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-kappa B. J Biol Chem 277(13):11570–11575. doi:10.1074/jbc.M112208200
Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem 277(24):21119–21122. doi:10.1074/jbc.C200179200
Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C (2015) NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 6:262. doi:10.3389/fphar.2015.00262
Jo EK, Kim JK, Shin DM, Sasakawa C (2016) Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13(2):148–159. doi:10.1038/cmi.2015.95
Hornung V, Latz E (2010) Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol 40(3):620–623. doi:10.1002/eji.200940185
Perregaux D, Gabel CA (1994) Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem 269(21):15195–15203
Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I (2012) ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis 3:e403. doi:10.1038/cddis.2012.144
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9(8):847–856. doi:10.1038/ni.1631
Elliott EI, Sutterwala FS (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265(1):35–52. doi:10.1111/imr.12286
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11(2):136–140. doi:10.1038/ni.1831
Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nunez G, He Y, Yin XM, O’Riordan MX (2015) Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 43(3):451–462. doi:10.1016/j.immuni.2015.08.008
Janssens S, Pulendran B, Lambrecht BN (2014) Emerging functions of the unfolded protein response in immunity. Nat Immunol 15(10):910–919. doi:10.1038/ni.2991
Shin S, Argon Y (2015) Stressed-out endoplasmic reticulum inflames the mitochondria. Immunity 43(3):409–411. doi:10.1016/j.immuni.2015.08.027
Hanamsagar R, Hanke ML, Kielian T (2012) Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol 33(7):333–342
Lampron A, Elali A, Rivest S (2013) Innate immunity in the CNS: redefining the relationship between the CNS and its environment. Neuron 78(2):214–232
Giambartolomei GH, Wallach JC, Baldi PC (2008) Neurobrucellosis. In: Halperin J (ed) Encephalitis: diagnosis and treatment. The Egerton Group, New York, pp. 255–272
Garcia Samartino C, Delpino MV, Pott Godoy C, Di Genaro MS, Pasquevich KA, Zwerdling A, Barrionuevo P, Mathieu P, Cassataro J, Pitossi F, Giambartolomei GH (2010) Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. Am J Pathol 176(3):1323–1338
Lee KM, Chiu KB, Sansing HA, Didier PJ, Ficht TA, Arenas-Gamboa AM, Roy CJ, Maclean AG (2013) Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Front Cell Infect Microbiol 3:86
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36(2):180–190
Aloisi F (2001) Immune function of microglia. Glia 36(2):165–179
Miraglia MC, Scian R, Samartino CG, Barrionuevo P, Rodriguez AM, Ibanez AE, Coria LM, Velasquez LN, Baldi PC, Cassataro J, Delpino MV, Giambartolomei GH (2013) Brucella abortus induces TNF-alpha-dependent astroglial MMP-9 secretion through mitogen-activated protein kinases. J Neuroinflammation 10:47
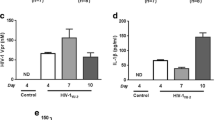
Miraglia MC, Costa Franco MM, Rodriguez AM, Bellozi PMQ, Ferrari CC, Farias MI, Dennis VA, Barrionuevo P, de Oliveira ACP, Pitossi F, Kim KS, Delpino MV, Oliveira SC, Giambartolomei GH (2016) Glial cell-elicited activation of brain microvasculature in 2 response to Brucella abortus infection requires ASC 3 inflammasome-dependent IL-1β production. J Immunol 196(9):3794–3805
Gosselin D, Rivest S (2007) Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain Behav Immun 21(3):281–289
Rosenzweig HL, Planck SR, Rosenbaum JT (2011) NLRs in immune privileged sites. Curr Opin Pharmacol 11(4):423–428
Delpino MV, Barrionuevo P, Macedo GC, Oliveira SC, Genaro SD, Scian R, Miraglia MC, Fossati CA, Baldi PC, Giambartolomei GH (2012) Macrophage-elicited osteoclastogenesis in response to Brucella abortus infection requires TLR2/MyD88-dependent TNF-alpha production. J Leukoc Biol 91(2):285–298
Barrionuevo P, Cassataro J, Delpino MV, Zwerdling A, Pasquevich KA, Garcia Samartino C, Wallach JC, Fossati CA, Giambartolomei GH (2008) Brucella abortus inhibits major histocompatibility complex class II expression and antigen processing through interleukin-6 secretion via Toll-like receptor 2. Infect Immun 76(1):250–262
McLean DR, Russell N, Khan MY (1992) Neurobrucellosis: clinical and therapeutic features. Clin Infect Dis 15(4):582–590
Alba D, Torres E, Molina F, Vazquez JJ (1992) Neutrophilic pleocytosis in brucella meningitis. Med Clin (Barc) 99(12):478
Lapaque N, Takeuchi O, Corrales F, Akira S, Moriyon I, Howard JC, Gorvel JP (2006) Differential inductions of TNF-alpha and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell Microbiol 8(3):401–413. doi:10.1111/j.1462-5822.2005.00629.x
Lapaque N, Muller A, Alexopoulou L, Howard JC, Gorvel JP (2009) Brucella abortus induces Irgm3 and Irga6 expression via type-I IFN by a MyD88-dependent pathway, without the requirement of TLR2, TLR4, TLR5 and TLR9. Microb Pathog 47(6):299–304. doi:10.1016/j.micpath.2009.09.005
Radhakrishnan GK, Yu Q, Harms JS, Splitter GA (2009) Brucella TIR domain-containing protein mimics properties of the Toll-like receptor adaptor protein TIRAP. J Biol Chem 284(15):9892–9898. doi:10.1074/jbc.M805458200
Voth DE, Broederdorf LJ, Graham JG (2012) Bacterial type IV secretion systems: versatile virulence machines. Future Microbiol 7(2):241–257. doi:10.2217/fmb.11.150
Burns DL (2003) Type IV transporters of pathogenic bacteria. Curr Opin Microbiol 6(1):29–34
Terradot L, Waksman G (2011) Architecture of the Helicobacter pylori Cag-type IV secretion system. FEBS J 278(8):1213–1222. doi:10.1111/j.1742-4658.2011.08037.x
Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A, Cazevieille C, Lavigne JP, Liautard JP, Ramuz M, O’Callaghan D (2002) Type IV secretion and Brucella virulence. Vet Microbiol 90(1–4):341–348
Comerci DJ, Martinez-Lorenzo MJ, Sieira R, Gorvel JP, Ugalde RA (2001) Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell Microbiol 3(3):159–168
den Hartigh AB, Sun YH, Sondervan D, Heuvelmans N, Reinders MO, Ficht TA, Tsolis RM (2004) Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect Immun 72(9):5143–5149. doi:10.1128/IAI.72.9.5143-5149.2004
Roux CM, Rolan HG, Santos RL, Beremand PD, Thomas TL, Adams LG, Tsolis RM (2007) Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell Microbiol 9(7):1851–1869. doi:10.1111/j.1462-5822.2007.00922.x
Acknowledgments
This study was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG), CNPq/CONICET, CAPES/PVE, CAPES/PNPD, CNPq/CT-Biotec, CNPq/CBAB, Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT-Argentina), Universidad de Buenos Aires, and National Institute of Health R01 AI116453.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on Dendritic Cell Subsets and Immune-mediated Diseases - Guest Editor: Francisco Quintana
Fernanda M. Marim and Miriam M. Costa Franco contributed equally to this study
Rights and permissions
About this article
Cite this article
Marim, F.M., Franco, M.M.C., Gomes, M.T.R. et al. The role of NLRP3 and AIM2 in inflammasome activation during Brucella abortus infection. Semin Immunopathol 39, 215–223 (2017). https://doi.org/10.1007/s00281-016-0581-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-016-0581-1