Abstract
Background
The recommended dose of erlotinib is 150 mg daily either 1 h before a meal (complete fasting) or 2 h after a meal (2 h post-meal), because of the food effect.
Methods
We conducted a cross-over pharmacokinetic study to compare the fed bioequivalence in the two conditions.
Results
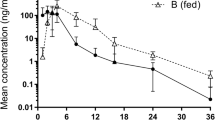
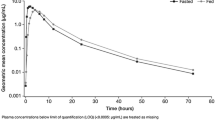
Twenty-three patients with non-small cell lung cancer were included in the analysis. AUC0–24 and C max in the 2-h post-meal status were significantly higher than in the complete fasting status (GMR = 1.33, P < 0.001; GMR = 1.44, P < 0.001, respectively). However, because the concentration of erlotinib did not reach the steady state within 7 days in the complete fasting state, we conducted analyses only on day 14, which showed no significant difference in AUC0–24 or C max between the two conditions. The more rapid increase in AUC0–24 and C min did not produce any earlier and more severe toxic events.
Conclusion
The AUC0–24 increased significantly faster (48–53 % greater) in the 2-h post-meal status than in complete fasting status, which suggested that the two gastric emptying states might differ in their absorption. However, there was no clinically significant difference in bioavailability or toxicity between the two clinically used fed conditions at least in 14 days.


Similar content being viewed by others
References
Mathijssen RH, Sparreboom A, Verweij J (2014) Determining the optimal dose in the development of anticancer agents. Nat Rev Clin Oncol 11(5):272–281
Szmulewitz RZ, Ratain MJ (2013) Playing Russian roulette with tyrosine kinase inhibitors. Clin Pharmacol Ther 93(3):242–244
Singh BN, Malhotra BK (2004) Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet 43(15):1127–1156
Ratain MJ (2011) Flushing oral oncology drugs down the toilet. J Clin Oncol 29(30):3958–3959
Sparreboom, A. and S.D. Baker, Pharmacokinetic studies in early anticancer drug development in Principles of Anticancer Drug Development. 2011: Humana Press/Springer Science
Ling J et al (2008) Effect of food on the pharmacokinetics of erlotinib, an orally active epidermal growth factor receptor tyrosine-kinase inhibitor, in healthy individuals. Anticancer Drugs 19(2):209–216
Yamamoto N et al (2008) Phase I dose-finding and pharmacokinetic study of the oral epidermal growth factor receptor tyrosine kinase inhibitor Ro50-8231 (erlotinib) in Japanese patients with solid tumors. Cancer Chemother Pharmacol 61(3):489–496
Hidalgo M et al (2001) Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 19(13):3267–3279
Soreide E et al (1996) Gastric emptying of a light hospital breakfast. A study using real time ultrasonography. Acta Anaesthesiol Scand 40(5):549–553
Tsuchiya K et al (2014) Low raltegravir concentration in cerebrospinal fluid in patients with ABCG2 genetic variants. J Acquir Immune Defic Syndr 66(5):484–486
Lu JF et al (2006) Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther 80(2):136–145
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458
Hamilton M et al (2006) Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res 12(7 Pt 1):2166–2171
Nakagawa K et al (2012) Postmarketing surveillance study of erlotinib in Japanese patients with non-small-cell lung cancer (NSCLC): an interim analysis of 3488 patients (POLARSTAR). J Thorac Oncol 7(8):1296–1303
Fukudo M et al (2013) Population pharmacokinetics/pharmacodynamics of erlotinib and pharmacogenomic analysis of plasma and cerebrospinal fluid drug concentrations in Japanese patients with non-small cell lung cancer. Clin Pharmacokinet 52(7):593–609
Rudin CM et al (2008) Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol 26(7):1119–1127
Fujita K et al (2014) High exposure to erlotinib and severe drug-induced interstitial lung disease in patients with non-small-cell lung cancer. Lung Cancer 86(1):113–114
Acknowledgments
We wish to thank all the patients who participated in this study and their families. We also wish to thank the staff of Department of Thoracic Oncology, National Cancer Center Hospital. This study was supported by the National Cancer Center Research and Development Fund (23-A-16) from the Ministry of Health, Labor, and Welfare of Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 5 and Figs. 3, 4 and 5.
Temporal changes in plasma trough concentrations (C min) of erlotinib in each cohort. On day 7, C min in cohort A was significantly higher than in cohort B (P = 0.017), though there was no significant difference on day 14 (P = 0.653). The trough plasma concentration of erlotinib had reached within 7 days only in 2-h post-meal condition. Box-and-whisker plots: The bottom and top of the box are the first and third quartiles, and the band inside the box is the second quartile (the median). The ends of the whiskers represent the lowest datum still within 1.5 interquartile range (IQR) of the lower quartile and the highest datum still within 1.5 IQR of the upper quartile
Rights and permissions
About this article
Cite this article
Katsuya, Y., Fujiwara, Y., Sunami, K. et al. Comparison of the pharmacokinetics of erlotinib administered in complete fasting and 2 h after a meal in patients with lung cancer. Cancer Chemother Pharmacol 76, 125–132 (2015). https://doi.org/10.1007/s00280-015-2778-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-015-2778-8