Abstract
Purpose
We conducted a phase I study for adjuvant chemotherapy of gemcitabine (GEM) plus S-1 in order to determine the maximum tolerated dose and the recommended dose (RD) and to evaluate the efficacy and toxicity of the regimen in curatively resected patients with biliary cancer.
Methods
The study included 34 patients with adequate organ functions, Eastern Cooperative Oncology Group PS 0-1, under 80 years of age, who had curative resection after August, 2007. Patients received GEM on day 1 and day 15, and S-1 from day 1 to day 14. Dose-limiting toxicities were determined during first two treatment cycles. After determining RD, a feasibility study was continued in the following four treatment cycles.
Results
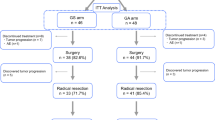
Hematological toxicity, particularly neutropenia and thrombocytopenia, was the most pronounced toxic effect of gemcitabine and S-1 adjuvant combination chemotherapy. The RD after pancreatoduodenectomy is GEM 1,000 mg/m2 + S-1 80 mg/m2, and RD after hemihepatectomy is GEM 800 mg/m2 + S-1 60 mg/m2.
Conclusions
The pharmacokinetics of GEM and S-1 indicate that changing the dose of adjuvant chemotherapy based on the operation method for biliary cancers is reasonable. We believe that this regimen will be established as an effective adjuvant chemotherapy for biliary cancer in the future.


Similar content being viewed by others
References
Sobin LH, Wittekind CH (eds) (2002) UICC TNM Classification of malignant tumors. Liver. 6th edn. Wiley-Liss, New York, pp 81–92
De Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM (1999) Biliary tract cancers. New Engl J Med 341:1368–1378
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Okusaka T (2002) Chemotherapy for biliary tract cancer in Japan. Semin Oncol 29:51–53
Patel T (2002) Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2:10
Shaib Y, El-Serag HB (2004) The epidemiology of cholangiocrcinoma. Semin Liver Dis 24:115–125
National Cancer Center (2007) Cancer statistics in Japan. http://www.fpcr.or.jp/publivation/statistics.html
Jarnagin WR, Fong Y, De Matteo RP, Gonen M, Burke EC, Bodniewicz BSJ, Youssef BAM, Klimstra D, Blumgart LH (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234:507–517
Nimura Y, Kamiya J, Kondo S, Nagino M, Uesaka K, Oda K, Sano T, Yamamoto H, Hayakawa N (2000) Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 7:155–162
Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, Nagakawa T, Nakayama T; Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract (2002) Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 95:1685–1695
Nakeeb A, Tran KQ, Black MJ, Erickson BA, Ritch PS, Quebbeman EJ, Wilson SD, Demeure MJ, Rilling WS, Dua KS, Pitt HA (2002) Improved survival in resected biliary malignancies. Surgery 132:555–564
Nelson JW, Ghofoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, Hurwitz HI, Bendell JC, Morse MA, Clough RW, Czito BG (2009) Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 73:148–153
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, Nakashima A, Sueda T (2009) Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 250(6):950–956
Eng C, Ramanathan RK, Wong MK, Remick SC, Dai L, Wade-Oliver KT, Mani S, Kindler HL (2004) A phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am J Clin Oncol 27:565–569
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical traials. Br J Cancer 96:896–902
Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S, Gayet B, Lotz JP, de Gramont A, Louvet C, GERCOR Group (2004) Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 15:1339–1343
Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ (2005) Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 23:2332–2338
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K, ACTS-GC Group (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, Nasu J, Ohtsu Ohtsu, Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomized phase 3 study. Lancet Oncol 10:1063–1069
Morizane C, Okusaka T, Furuse J, Ishii H, Ueno H, Ikeda M, Nakachi K, Najima M, Ogura T, Suzuki E (2009) A phase II study of S-1 in gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol 63:313–319
Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T, Funakoshi A (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62:849–855
Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C (2004) Phase II study of S-1 in patients with advanced biliary tract cancer. Br J Cancer 91:1769–1774
Valle JW, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABC-02 Trial Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J, Koshiji M, Nambu Y, Furuse J, Miyazaki M, Nimura Y (2010) Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103:469–474
Nakamura K, Yamaguchi T, Ishihara T, Sudo K, Kato H, Saisho H (2006) Phase II trial of oral S-1 combined with gemcitabine in metastatic pancreatic cancer. Br J Cancer 94:1575–1579
Nakai Y, Isayama H, Sasaki T, Sasahira N, Ito Y, Kogure H, Togawa O, Matsubara S, Arizumi T, Yagioka H, Yashima Y, Kawakubo K, Mizuno S, Yamamoto K, Hirano K, Tsujino T, Ijichi H, Toda N, Tada M, Kawabe T, Omata M (2009) A pilot study for combination chemotherapy using gemcitabine and S-1 for advanced pancreatic cancer. Oncology 77(5):300–303
Sasaki T, Isayama H, Nakai Y, Ito Y, Kogure H, Togawa O, Toda N, Yasuda I, Hasebe O, Maetani I, Sasahira N, Hirano K, Tsujino T, Tada M, Omata M (2010) Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmarcol 65:1101–1107
Kanai M, Yoshimura K, Tsumura T, Asada M, Suzuki C, Niimi M, Matsumoto S, Nishimura T, Nitta T, Yasuchika K, Taura K, Mori Y, Hamada A, Inoue N, Tada S, Yanagihara K, Yazumi S, Osaki Y, Chiba T, Ikai I, Fukushima M, Uemoto S, Hatano E (2011) A multi-institution phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother Pharmarcol 67:1429–1434
Takashima A, Morizane C, Ishii H, Nakamura K, Fukuda H, Okusaka T, Furuse J (2010) Randomized phase II study of gemcitabine plus S-1 combination therapy versus S-1 in advanced biliary tract cancer: Japan Clinical Oncology Group Study (JCOG0805). Jpn J Clin Oncol 40:1189–1191
Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, Nakashima A, Sueda T (2009) Gemcitabine-based adjuvant chemotherapy improved survival after aggressive surgery for hilar cholangiocarcinoma. J Gastrointest Surg 13:1470–1479
Ren Q, Kao V, Grem JL (1998) Cytotoxicity and DNA fragmentation associated with sequential gemcitabine and 5-fluoro-2′-deoxyuridine in HT-29 colon cancer cells. Clin Cancer Res 4:2811–2818
Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA III, Green MR, Tarassoff PG, Brown TD, Casper ES, Storniolo AM, Von Hoff DD (1996) A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol 7:347–353
Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, Plunkett W (1990) Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol Pharmocol 38:567–572
Madajewicz S, Hentschel P, Burns P, Caruso R, Fiore J, Fried M, Malhotra H, Ostrow S, Sugarman S, Viola M (2000) Phase I chemotherapy study of biochemical modulation of folinic acid and fluorouracil by gemcitabine in patients with solid tumor malignancies. J Clin Oncol 18:3553–3557
Pressacco J, Mirovski B, Erlichman C, Hedley DW (1995) Effects of thymidylate synthase inhibition on thymidine kinase activity and nucleoside transporter expression. Cancer Res 55:1505–1508
Nakahira S, Nakamori S, Tsujie M, Takeda S, Sugimoto K, Takahashi Y, Okami J, Marubashi S, Miyamoto A, Takeda Y, Nagano H, Dono K, Umeshita K, Sakon M, Monden M (2008) Pretreatment with S-1, an oral derivative of 5-fluorouracil, enhances gemcitabine effects in pancreatic cancer xenografts. Anticancer Res 28:179–186
Floyd FJ, Hornbeck CL, Byfield JE, Griffiths JC, Frankel SS (1982) Clearance of continuously infused 5-fluoraouracil in adults having lung or gastrointestinal carcinoma with or without hepatic metastases. Drug Intell Clin Pharm 16:665–667
Yoon DH, Lee HJ, Hong YS, Kim K, Lee SS, Lee JL, Chang HM, Ryu MH, Kang YK, Lee JS, Kim TW (2011) A phase I study of S-1 treatment with a 3 week schedule in advanced biliary cancer with or without hepatic dysfunction. Invest New Drugs 29:332–339
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahara, T., Nitta, H., Hasegawa, Y. et al. A phase I study for adjuvant chemotherapy of gemcitabine plus S-1 in curatively resected patients with biliary tract cancer: adjusting the dose of adjuvant chemotherapy according to the surgical procedures. Cancer Chemother Pharmacol 69, 1127–1133 (2012). https://doi.org/10.1007/s00280-011-1805-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1805-7