Abstract
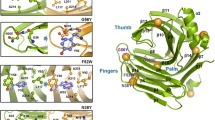
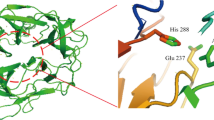
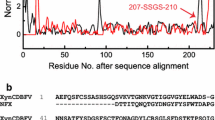
XynCDBFV from Neocallimastix patriciarum is among the most effective xylanases and holds great potentials in a wide variety of industrial applications. In the present study, several active site residues were modified referring to the instrumental information of the complex structure of XynCDBFV and xylooligosaccharides. Among the 12 single active site mutants, W125F and F163W show increased activity comparing to the wild-type protein. The double mutant W125F/F163W was then generated which displayed nearly 20 % increase in the enzyme activity. Although W125F/F163W showed 5 °C reduction in the optimal temperature, it still preserves similar thermostability and is more active than the wild-type enzyme at temperatures lower than 60 °C. These properties make the double mutant a suitable candidate for commercial applications that involve lower operating temperatures. Furthermore, we investigated the effect of N-glycosylation on the thermostability of XynCDBFV when expressed in the yeast strain Pichia pastoris for industrial use. Two potential glycosylation sites (Asn-37 and Asn-88) were examined, and their roles in enzyme performance were validated. We found that the N-glycosylations of XynCDBFV are related to both catalytic activity and heat stability, with Asn-37 motif playing a dominant role. Collectively, the enzymatic properties of XynCDBFV were improved by molecular engineering, and the influences of N-glycosylations on the enzyme have been clearly elucidated herein.





Similar content being viewed by others
References
Beckham GT, Dai Z, Matthews JF, Momany M, Payne CM, Adney WS, Baker SE, Himmel ME (2012) Harnessing glycosylation to improve cellulase activity. Curr Opin Biotechnol 23:338–345. doi:10.1016/j.copbio.2011.11.030
Biely P, Vrsanska M, Tenkanen M, Kluepfel D (1997) Endo-β-1,4-xylanase families: differences in catalytic properties. J Biotechnol 57:151–166
Bornscheuer UT, Pohl M (2001) Improved biocatalysts by directed evolution and rational protein design. Curr Opin Chem Biol 5:137–143
Böttcher D, Bornscheuer UT (2010) Protein engineering of microbial enzymes. Curr Opin Microbiol 13:274–282. doi:10.1016/j.mib.2010.01.010
Bretthauer RK, Castellino FJ (1999) Glycosylation of Pichia pastoris-derived proteins. Biotechnol Appl Biochem 30(Pt 3):193–200
Chen YL, Tang TY, Cheng KJ (2001) Directed evolution to produce an alkalophilic variant from a Neocallimastix patriciarum xylanase. Can J Microbiol 47:1088–1094
Cheng YS, Ko TP, Huang JW, Wu TH, Lin CY, Luo W, Li Q, Ma Y, Huang CH, Wang AH, Liu JR, Guo RT (2012) Enhanced activity of Thermotoga maritima cellulase 12A by mutating a unique surface loop. Appl Microbiol Biotechnol 95:661–669. doi:10.1007/s00253-011-3791-4
Cheng YS, Chen CC, Huang CH, Ko TP, Luo W, Huang JW, Liu JR, Guo RT (2014) Structural analysis of a glycoside hydrolase family 11 xylanase from Neocallimastix patriciarum: insights into the molecular basis of a thermophilic enzyme. J Biol Chem 289:11020–11028. doi:10.1074/jbc.M114.550905
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23. doi:10.1016/j.femsre.2004.06.005
De Lemos EF, Gouders T, Lamotte-Brasseur J, Rigali S, Frere JM (2005) Improving the alkalophilic performances of the Xyl1 xylanase from Streptomyces sp. S38: structural comparison and mutational analysis. Protein Sci 14:292–302. doi:10.1110/ps.04978705
Fonseca-Maldonado R, Vieira DS, Alponti JS, Bonneil E, Thibault P, Ward RJ (2013) Engineering the pattern of protein glycosylation modulates the thermostability of a GH11 xylanase. J Biol Chem 288:25522–25534. doi:10.1074/jbc.M113.485953
Gao F, Jiang Y, Zhou GH, Han ZK (2007) The effects of xylanase supplementation on growth, digestion, circulating hormone and metabolite levels, immunity and gut microflora in cockerels fed on wheat-based diets. Br Poult Sci 48:480–488. doi:10.1080/00071660701477320
Huang JW, Cheng YS, Ko TP, Lin CY, Lai HL, Chen CC, Ma Y, Zheng Y, Huang CH, Zou P, Liu JR, Guo RT (2012) Rational design to improve thermostability and specific activity of the truncated Fibrobacter succinogenes 1,3-1,4-β-D-glucanase. Appl Microbiol Biotechnol 94:111–121. doi:10.1007/s00253-011-3586-7
Huang JW, Chen CC, Huang CH, Huang TY, Wu TH, Cheng YS, Ko TP, Lin CY, Liu JR, Guo RT (2014) Improving the specific activity of β-mannanase from Aspergillus niger BK01 by structure-based rational design. Biochim Biophys Acta 1844:663–669. doi:10.1016/j.bbapap.2014.01.011
Joshi MD, Sidhu G, Pot I, Brayer GD, Withers SG, McIntosh LP (2000) Hydrogen bonding and catalysis: a novel explanation for how a single amino acid substitution can change the pH optimum of a glycosidase. J Mol Biol 299:255–279. doi:10.1006/jmbi.2000.3722
König J, Grasser R, Pikor H, Vogel K (2002) Determination of xylanase, β-glucanase, and cellulase activity. Anal Bioanal Chem 374:80–87. doi:10.1007/s00216-002-1379-7
Labrou NE (2010) Random mutagenesis methods for in vitro directed enzyme evolution. Curr Protein Pept Sci 11:91–100
Lu D, Yang C, Liu Z (2012) How hydrophobicity and the glycosylation site of glycans affect protein folding and stability: a molecular dynamics simulation. J Phys Chem B 116:390–400. doi:10.1021/jp203926r
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Otten LG, Hollmann F, Arends IW (2010) Enzyme engineering for enantioselectivity: from trial-and-error to rational design? Trends Biotechnol 28:46–54. doi:10.1016/j.tibtech.2009.10.001
Paes G, O’Donohue MJ (2006) Engineering increased thermostability in the thermostable GH-11 xylanase from Thermobacillus xylanilyticus. J Biotechnol 125:338–350. doi:10.1016/j.jbiotec.2006.03.025
Pokhrela S, Joo JC, Lee HS, Yoo YJ (2013) Activity enhancement of a Bacillus circulans xylanase by introducing ion-pair interactions into an α-helix. Process Biochem 48:1495–1501
Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67:577–591. doi:10.1007/s00253-005-1904-7
Ramalingam ADHaC (2010) Xylanases and its application in food industry: a review. J Exp Sci 1:1-11
Romero PA, Arnold FH (2009) Exploring protein fitness landscapes by directed evolution. Nat Rev Mol Cell Biol 10:866–876. doi:10.1038/nrm2805
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291. doi:10.1007/s10295-003-0049-x
Sapag A, Wouters J, Lambert C, de Ioannes P, Eyzaguirre J, Depiereux E (2002) The endoxylanases from family 11: computer analysis of protein sequences reveals important structural and phylogenetic relationships. J Biotechnol 95:109–131 doi:10.1016/S0168-1656(02)00002–0
Shental-Bechor D, Levy Y (2008) Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc Natl Acad Sci U S A 105:8256–8261. doi:10.1073/pnas.0801340105
Song L, Siguier B, Dumon C, Bozonnet S, O’Donohue MJ (2012) Engineering better biomass-degrading ability into a GH11 xylanase using a directed evolution strategy. Biotechnol Biofuels 5:3. doi:10.1186/1754-6834-5-3
Sriprang R, Asano K, Gobsuk J, Tanapongpipat S, Champreda V, Eurwilaichitr L (2006) Improvement of thermostability of fungal xylanase by using site-directed mutagenesis. J Biotechnol 126:454–462. doi:10.1016/j.jbiotec.2006.04.031
Stephens DE, Singh S, Permaul K (2009) Error-prone PCR of a fungal xylanase for improvement of its alkaline and thermal stability. FEMS Microbiol Lett 293:42–47. doi:10.1111/j.1574-6968.2009.01519.x
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol 22:33–64. doi:10.1080/07388550290789450
Umemoto H, Ihsanawati IM, Yatsunami R, Fukui T, Kumasaka T, Tanaka N, Nakamura S (2009) Improvement of alkaliphily of Bacillus alkaline xylanase by introducing amino acid substitutions both on catalytic cleft and protein surface. Biosci Biotechnol Biochem 73:965–967
Viikari L, Kantelinen A, Sundquist J, Linko M (1994) Xylanases in bleaching: from an idea to the industry. FEMS Microbiol Rev 13:335–350
Wu TH, Chen CC, Cheng YS, Ko TP, Lin CY, Lai HL, Huang TY, Liu JR, Guo RT (2014) Improving specific activity and thermostability of Escherichia coli phytase by structure-based rational design. J Biotechnol 175:1–6. doi:10.1016/j.jbiotec.2014.01.034
You C, Yuan H, Huang Q, Lu H (2010) Substrate molecule enhances the thermostability of a mutant of a family 11 xylanase from Neocallimastix patriciarum. Afr J Biotechnol 9:1288–1294
Zou S, Xie L, Liu Y, Kaleem I, Zhang G, Li C (2012) N-linked glycosylation influences on the catalytic and biochemical properties of Penicillium purpurogenum β-D-glucuronidase. J Biotechnol 157:399–404. doi:10.1016/j.jbiotec.2011.12.017
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2011CB710800 and 2011CBA00805), the National High Technology Research and Development Program of China (2012AA022200), the National Natural Science Foundation of China (31200053 and 31300615), and the Chinese Academy of Sciences (KSZD-EW-Z-015–2).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ya-Shan Cheng and Chun-Chi Chen contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 51 kb)
Rights and permissions
About this article
Cite this article
Cheng, YS., Chen, CC., Huang, JW. et al. Improving the catalytic performance of a GH11 xylanase by rational protein engineering. Appl Microbiol Biotechnol 99, 9503–9510 (2015). https://doi.org/10.1007/s00253-015-6712-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6712-0