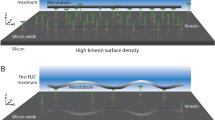
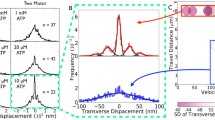
Abstract
Although the velocity of single kinesin motors against an opposing force F of 0–10 pN is well known, the behavior of multiple kinesin motors working to overcome a larger load is still poorly understood. We have carried out gliding assays in which 3–7 Drosophila kinesin-1 motors moved a microtubule at 200–700 μm/s against a 0–31 pN load at saturating [ATP]. The load F was generated by applying a spatially uniform magnetic field gradient to a superparamagnetic bead attached to the (+) end of the microtubule. When F was scaled by the average number of motors 〈n〉, the force–velocity relationship for multiple motors was similar to the force–velocity relationship for a single motor, supporting a minimal load-sharing model. The velocity distribution at low load has a single mode consistent with rapid fluctuations of n. However, against a load of 2.5–4.7 pN/motor, additional modes appeared at lower velocity. These observations support the Klumpp–Lipowsky model of multimotor transport [Proc Natl Acad Sci USA 102. 17284–17289 (2005)].






Similar content being viewed by others
Abbreviations
- SEM:
-
Standard error of the mean
- BRB80:
-
Brinkley reconstitution buffer
References
Beeg J, Klumpp S, Dimova R, Garcia RS, Unger E (2008) Transport of beads by several kinesin motors. Biophys J 94:532–541
Bevington PR (1969) Data reduction and error analysis for the physical sciences. McGraw-Hill, New York
Bieling P, Telley IA, Piehler J, Surrey T (2008) Processive kinesins require loose mechanical coupling for efficient collective motility. EMBO Reports 1–7
Block SM, Goldstein LSB, Schnapp BJ (1990) Bead movement by single kinesin molecules studied with optical tweezers. Nature 348:348–352
Chisena EN, Wall RA, Macosko JC, Holzwarth GM (2007) Speckled microtubules improve tracking in motor-protein gliding assays. Phys Biol 7:10–15
Coppin CM, Pierce DW, Hsu L, Vale RD (1997) The load dependence of kinesin’s mechanical cycle. Proc Natl Acad Sci USA 94:8539–8544
Coy DL, Wagenbach M, Howard J (1999) Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J Biol Chem 274:3667–3671
Fallesen TL (2010) Kinesin-microtubule interactions during gliding assays under magnetic force physics. Wake Forest University, Winston-Salem, pp 193, http://hdl.handle.net/10339/30429
Fallesen TL, Hill DB, Steen M, Macosko JC, Bonin KGH (2010) Magnet polepiece design for uniform magnetic force on superparamagnetic beads. Rev Sci Inst 81:074303
Fallesen TL, Macosko JC, Holzwarth G (2011) Measuring the number and spacing of molecular motors propelling a gliding microtubule. Phys Rev E 83:011918, http://link.aps.org/doi/10.1103/PhysRevE.83.011918
Forstner W (1994) Image matching. In: Haralick RM, Shapiro LG (eds) Computer and robot vision, vol 2. Addison-Wesley, Reading, MA, pp 289–378
Gagliano J, Walb M, Blaker B, Macosko JC, Holzwarth G (2010) Kinesin velocity increases with the number of motors pulling against viscoelastic drag. Eur Biophys J 39:801–813
Gibbons F, Chauwin J-F, Desposito M, Jose JV (2001) A dynamic model of kinesin-microtublue motility assays. Biophys J 80:2515–2526
Hill DB, Plaza MJ, Bonin KD, Holzwarth G (2004) Fast Vesicle Transport in PC12 Neurites: velocities and forces. Eur Biophys J 33:623–632
Hirokawa N, Noda Y, Tanaka Y, Niwa S (2009) Kinesin superfamily motor proteins and intracellullar transport. Nat Rev Mol Cell Biol 10:662–696
Howard J (2001) Mechanics of motor proteins and the cytoskeleton. Sinauer Associates, Sunderland, MA
Howard J, Hudspeth AJ, Vale RD (1989) Movement of microtubules by single kinesin molecules. Nature 342:154–158
Hunt AJ, Gittes F, Howard J (1994) The force exerted by a single kinesin molecule against a viscous load. Biophys J 67:766–781
Jamison DK, Driver JW, Rogers AR, Constantinou PE, Diehl MR (2010) Two kinesins transport cargo primarily via the action of one motor: implications for intracellular transport. Biophys J 99:2967–2977
Katira P, Agarwal A, Fischer T, Chen H-Y, Jiang X, Lahann J, Hess H (2007) Quantifying the performance of protein-resisting surfaces at ultra-low protein coverages using kinesin motor proteins as probes. Adv Mater 19:3171–3176
Klumpp S, Lipowsky R (2005) Cooperative cargo transport by several molecular motors. Proc Natl Acad Sci USA 102:17284–17289
Kojima H, Muto E, Higuchi H, Yanagida T (1997) Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys J 73:2012–2022
Korn CB, Klumpp S, Lipowsky R, Schwarz US (2009) Stochastic simulations of cargo transport by processive molecular motors. J Chem Phys 131:245107
Kunwar A, Mogilner A (2010) Robust transport by multiple motors with nonlinear force-velocity relations and stochastic load sharing. Phys Biol 7:016012
Leduc C, Campas O, Zeldovich KB, Roux A, Jolimaitre P, Borel-Bonnet L, Goud B, Joanny J-F, Prost J (2004) Cooperative extraction of membrane nanotubes by molecular motors. Proc Natl Acad Sci USA 101:17096–17101
Leduc C, Ruhnow F, Howard J, Diez S (2007) Detection of fractional steps in cargo movement by the collective operation of kinesin-1 motors. Proc Natl Acad Sci USA 104:10847–10852
Meyhöfer E, Howard J (1995) The force generated by a single kinesin molecule against an elastic load. Proc Natl Acad Sci USA 92:574–578
Nan X, Sims PA, Xie XS (2008) Organelle tracking in a living cell with microsecond time resolution and nanometer spatial precision. ChemPhysChem 2008:707–712
Pincet F, Husson J (2005) The solution to the streptavidin-biotin paradox: the influence of history on the strength of single molecular bonds. Biophys J 89:4374–4381
Shtridelman Y, Holzwarth GM, Bauer CT, Gassman NR, DeWitt DA, Macosko JC (2009) In vivo multimotor force-velocity curves by tracking and sizing sub-diffraction limited vesicles. Cel Mol Bioeng 2:190–199
Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, Gross SP (2008) Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 135:1098–1107
Silverman BW (1986) Density estimation for statistics and data analysis, 1st edn. Chapman and Hall, London
Vale RD, Malik F, Brown D (1992) Directional instability of microtubule transport in the presence of kinesin and dynein, two opposite polarity motor proteins. J Cell Biol 119:1589–1596
Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP (2007) Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci USA 104:87–92
Visscher K, Schnitzer MJ, Block SM (1999) Single kinesin molecules studied with a molecular force clamp. Nature 400:184–189
Watanabe TM, Higuchi H (2007) Stepwise movements in vesicle transport of HER2 by motor proteins in living cells. Biophys J 92:4109–4120
Welte MA, Gross S, Postner M, Block S, Wieschaus E (1998) Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92:547–557
Yildiz A, Tomishige M, Vale RD, Selvin PR (2004) Kinesin walks hand-over-hand. Science 303:676–678
Acknowledgments
We thank J. Howard for providing plasmid pPK113 for kinesin-1, Jason Gagliano for assistance in the preparation of kinesin, and Matt Steen for assistance with Odyssey software. We are grateful for helpful suggestions from the reviewers. Support from NIH grant R15 NS053493 (G.H.) and Wake Forest University funds (J.C.M.) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Conversion of {x,y,t} tracks to {x″,y″,t} data for which x″ is parallel to the path of the microtubule and y″ is perpendicular to that path.
Consider a microtubule traveling at constant velocity in a straight path, x = x 0 + v x t, y = y 0 + v y t, with v x and v y independent of t.
Step 1. Translocate the (x,y,t) frame to a new coordinate frame (x′,y′,t) in which the microtubule starts at (0,0,0) with equations x′ = x − x0, y′ = y − y0. In the x′y′ plane, y′ = (v y /v x )x′, a straight line through the origin.
Step 2. Rotate the (x′,y′) coordinate system by angle θ = tan−1(v y /v x ) to new coordinates (x″,y″):\( \left[ {\begin{array}{*{20}c} {x^{\prime \prime} } \\ {y^{\prime \prime} } \\ \end{array} } \right] = \left[ {\begin{array}{*{20}c} {\cos (\theta )} & {\sin (\theta )} \\ { - \sin (\theta )} & {\cos (\theta )} \\ \end{array} } \right]\left[ {\begin{array}{*{20}c} {x^\prime } \\ {y^\prime } \\ \end{array} } \right]. \)
In the (x″,y″) coordinate system, \( x^{\prime\prime} = \left( {\sqrt {v_{x}^{2} + v_{y}^{2} } } \right)t = vt \) and y″ = 0. Thus, the slope \( \frac{{{\text{d}}x^{\prime \prime} }}{{{\text{d}}t}} \) equals the velocity of the particle.
For a microtubule traveling along a curved path, v x and v y are functions of t, so θ = tan−1(v y /v x ) is also a function of t. We determined θ(t) from the smoothed value of dy/dx. Steps 1 and 2 were then applied to data points 1 and 2. This done, steps 1 and 2 were applied to points 2 and 3, etc. (This is easy in MATLAB.) In that (x″,y″,t) coordinate system, y″(t) = 0 + noise, whereas x″(t) = vt + noise. We tested the algorithm carefully with synthetic data, i.e., data sets {x,y,t} constructed with known values of v x and v y, plus Gaussian noise.
Rights and permissions
About this article
Cite this article
Fallesen, T.L., Macosko, J.C. & Holzwarth, G. Force–velocity relationship for multiple kinesin motors pulling a magnetic bead. Eur Biophys J 40, 1071–1079 (2011). https://doi.org/10.1007/s00249-011-0724-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0724-1