Abstract
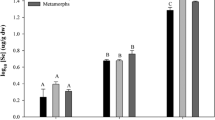
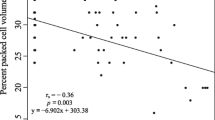
Selenium (Se) is an essential micronutrient with a narrow therapeutic concentration range. The relative toxicity of Se increases as it is biotransformed into organic compounds, primarily selenomethionine (SeMet), within the aquatic food chain. Effects of aquatic Se contamination are well quantified for many freshwater fish and aquatic bird species, but impacts on amphibians are not well known. This study investigated the responses of larval Cope’s gray tree frogs (Hyla chrysoscelis) fed a diet enriched with one of two concentrations of SeMet (50.1 and 489.9 μg Se g−1 dw [low and high groups, respectively]) by way of a food-limited (ration) or ad libitum (ad lib) feeding regimen. The high dose caused 100 % mortality during the larval period independent of resource provision levels. Regardless of feeding regimen, the low dose decreased larval survival and successful metamorphosis relative to control treatments. The low dose also induced rear limb deformities in ≤73 % of individuals initiating metamorphosis. Providing low-dose food by way of a rationed feeding regimen decreased observed toxicity, likely because of decreased dietary exposure to SeMet relative to the low ad lib treatment. Individuals from the low ration treatment had decreased wet mass at initiation and completion of metamorphic climax (Gosner stages 42 through 46) compared with those from the control ad lib treatment, indicating that resource limitation combined with Se exposure might negatively affect energy stores after metamorphosis. However, lipid content analyses of recently metamorphosed individuals did not reveal any influence of treatment or resource provision on energy stored as lipids. The mean tissue Se concentration of individuals that received the low dose and completed metamorphosis was significantly greater than that of control ad lib or ration individuals at the same developmental stage. This study demonstrates that larval exposure to dietary SeMet can decrease growth and survival and induce deformities in a developing amphibian. Furthermore, retention of Se body burdens through metamorphosis suggests that surviving individuals can transport Se accumulated from contaminated aquatic environments into terrestrial food webs.
Similar content being viewed by others
References
Brown HHK, Tyler HK, Mousseau TA (2004) Orajel as an amphibian anesthetic: refining the technique. Herpetol Rev 35:252
Fan TWM, Teh SJ, Hinton DE, Higashi RM (2002) Selenium biotransformations into proteinaceous forms by foodweb organisms of selenium-laden drainage waters in California. Aquat Toxicol 57:65–84
Fitzpatrick LC (1976) Life-history patterns of storage and utilization of lipids for energy in amphibians. Am Zool 16:725–732
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Goyer RA, Clarkson TW (2001) Toxic effects of metals. In: Klaassen CD (ed) Cassarett & Doull’s toxicology. McGraw-Hill, New York, pp 811–867
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Harvey HR, Pleuthner RL, Lessard EJ, Bernhardt MJ, Tracy Shaw C (2012) Physical and biochemical properties of the euphausiids Thysanoessa inermis, Thysanoessa raschii, and Thysanoessa longipes in the eastern Bering Sea. Deep-Sea Res II Top Stud Oceanogr 65–70:173–183
Heinz GH, Hoffman DJ, Gold LG (1988) Toxicity of organic and inorganic selenium to mallard ducklings. Arch Environ Contam Toxicol 17:561–568
Heinz GH, Hoffman DJ, LeCaptain LJ (1996) Toxicity of seleno-l-methionine, seleno-dl-methionine, high selenium wheat, and selenized yeast to mallard ducklings. Arch Environ Contam Toxicol 30:93–99
Hopkins WA, Mendonca MT, Rowe CL, Congdon JD (1998) Elevated trace element concentrations in southern toads, Bufo terrestris, exposed to coal combustion waste. Arch Environ Contam Toxicol 35:325–329
Hopkins WA, Congdon J, Ray JK (2000) Incidence and impact of axial malformations in larval bullfrogs (Rana catesbeiana) developing in sites polluted by a coal-burning power plant. Environ Toxicol Chem 19:862–868
Hopkins WA, Snodgrass JW, Roe JH, Staub BP, Jackson BP, Congdon JD (2002) Effects of food ration on survival and sublethal responses of lake chubsuckers (Erimyzon sucetta) exposed to coal combustion wastes. Aquat Toxicol 57:191–202
Hothem RL, Ohlendorf HM (1989) Contaminants in foods of aquatic birds at Kesterson Reservoir California USA 1985. Arch Environ Contam Toxicol 18:773–786
Janz DM, Deforest DK, Brooks ML, Chapman PM, Gilron G, Hoff D et al (2010) Selenium toxicity to aquatic organisms. In: Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA et al (eds) Ecological assessment of selenium in the aquatic environment. Society of Environmental Toxicology and Chemistry, Pensacola, pp 141–231
Ju SJ, Harvey HR (2004) Lipids as markers of nutritional condition and diet in the Antarctic krill Euphausia superba and Euphausia crystallorophias during austral winter. Deep-Sea Res II Top Stud Oceanogr 51:2199–2214
Lemly AD (1993) Metabolic stress during winter increases the toxicity of selenium to fish. Aquat Toxicol 27:133–158
Lemly AD (2002) Symptoms and implications of selenium toxicity in fish: the Belews Lake case example. Aquat Toxicol 57:39–49
Maier KJ, Foe CG, Knight AW (1993) Comparative toxicity of selenate, selenite, seleno-dl-methionine and seleno-dl-cystine to Daphnia magna. Environ Toxicol Chem 12:755–763
Ohlendorf HM (2002) The birds of Kesterson Reservoir: a historical perspective. Aquat Toxicol 57:1–10
Ohlendorf HM, Hothem RL, Aldrich TW (1988) Bioaccumulation of selenium by snakes and frogs in the San-Joaquin Valley, California. Copeia 3:704–710
Raimondo SM, Rowe CL, Congdon JD (1998) Exposure to coal ash impacts swimming performance and predator avoidance in larval bullfrogs (Rana catesbeiana). J Herpetol 32:289–292
Roe JH, Hopkins WA, Jackson BP (2005) Species- and stage-specific differences in trace element tissue concentrations in amphibians: implications for the disposal of coal-combustion wastes. Environ Pollut 136:353–363
Rosetta TN, Knight AW (1995) Bioaccumulation of selenate, selenite, and seleno-dl-methionine by the brine fly larvae Ephydra cinerea Jones. Arch Environ Contam Toxicol 29:351–357
Rowe CL, Kinney OM, Fiori AP, Congdon JD (1996) Oral deformities in tadpoles (Rana catesbeiana) associated with coal ash deposition: effects on grazing ability and growth. Freshw Biol 36:723–730
Rowe CL, Kinney OM, Nagle RD, Congdon JD (1998) Elevated maintenance costs in an anuran (Rana catesbeiana) exposed to a mixture of trace elements during the embryonic and early larval periods. Physiol Zool 71:27–35
Rowe CL, Hopkins WA, Coffman VR (2001) Failed recruitment of southern toads (Bufo terrestris) in a trace element-contaminated breeding habitat: direct and indirect effects that may lead to a local population sink. Arch Environ Contam Toxicol 40:399–405
Rowe CL, Hopkins WA, Congdon JD (2002) Ecotoxicological implications of aquatic disposal of coal combustion residues in the United States: a review. Environ Monit Assess 80:207–276
Rowe CL, Heyes A, Hopkins W (2009) Effects of dietary vanadium on growth and lipid storage in a larval anuran: results from studies employing ad libitum and rationed feeding. Aquat Toxicol 91:179–186
Rowe CL, Heyes A, Hilton J (2011) Differential patterns of accumulation and depuration of dietary selenium and vanadium during metamorphosis in the Gray Treefrog (Hyla versicolor). Arch Environ Contam Toxicol 60:336–342
Saiki MK, Lowe TP (1987) Selenium in aquatic organisms from subsurface agricultural drainage water San Joaquin Valley California USA. Arch Environ Contam Toxicol 16:657–670
Sawant VA, Varute AT (1973) Lipid changes in tadpoles of Rana tigrina during growth and metamorphosis. Comp Biochem Physiol 44:729–750
Schuler CA, Anthony RG, Ohlendorf HM (1990) Selenium in wetlands and waterfowl foods at Kesterson Reservoir California USA 1984. Arch Environ Contam Toxicol 19:845–853
Scott DE (1994) The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 75:1383–1396
Scott DE, Casey ED, Donovan MF, Lynch TK (2007) Amphibian lipid levels at metamorphosis correlate to post-metamorphic terrestrial survival. Oecologia 153:521–532
Segawa R (2008) Standard operating procedure: calculating pesticide concentration in dry and wet soil. METH006.00. California Department of Pesticide Regulation, Sacramento, CA
Snodgrass JW, Hopkins WA, Broughton J, Gwinn D, Baionno JA, Burger J (2004) Species-specific responses of developing anurans to coal combustion wastes. Aquat Toxicol 66:171–182
Thomas JK, Janz DM (2012) Dietary selenomethionine exposure in adult zebrafish alters swimming performance, energetics and the physiological stress response. Aquat Toxicol 102:79–86
Torreilles SL, McClure DE, Green SL (2009) Evaluation and refinement of euthanasia methods for Xenopus laevis. J Am Assoc Lab Animal Sci 48:512–516
United States Environmental Protection Agency (2004) Draft aquatic life water quality criteria for selenium. EPA 822-D-04-001. Office of Water, Office of Science and Technology, USEPA, Washington, DC
Unrine JM, Hopkins WA, Romanek CS, Jackson BP (2007) Bioaccumulation of trace elements in omnivorous amphibian larvae: implications for amphibian health and contaminant transport. Environ Pollut 149:182–192
Wilbur HM, Collins JP (1973) Ecological aspects of amphibian metamorphosis. Science 182:1305–1314
Young TF, Finley K, Adams WJ, Besser J, Hopkins WA, Jolley D et al (2010) What you need to know about selenium. In: Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA et al (eds) Ecological assessment of selenium in the aquatic environment. Society of Environmental Toxicology and Chemistry, Pensacola, pp 7–46
Acknowledgments
This research was conducted in accordance with University of Maryland Center for Environmental Science Institutional Animal Care and Use Committee approved protocol F-CBL-03-03. We gratefully acknowledge R. Pleuthner, R. Harvey, C. Mitchelmore, E. Jenny, K. Eisenreich, and E. Stefansson for their contributions to this work. Funding for this research was provided in part by an award from the University of Maryland, Baltimore, and from the Graduate Education Committee of the Chesapeake Biological Laboratory. Funding for this research was provided in part by awards from the University of Maryland, Baltimore and the Graduate Education Committee of the Chesapeake Biological Laboratory and contribution 4704 of the University of MD Center for Environmental Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lockard, L., Rowe, C.L. & Heyes, A. Dietary Selenomethionine Exposure Induces Physical Malformations and Decreases Growth and Survival to Metamorphosis in an Amphibian (Hyla chrysoscelis) . Arch Environ Contam Toxicol 64, 504–513 (2013). https://doi.org/10.1007/s00244-012-9850-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9850-8