Abstract
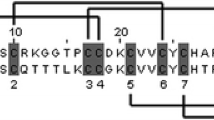
We report the cloning and sequence analysis of Echis ocellatus cDNAs coding for dimeric disintegrin subunits and for the short disintegrin ocellatusin. All the dimeric disintegrin subunit messengers belong to the short-coding class, indicating that short messengers may be more widely distributed than previously thought. Mass spectrometric analysis of the HPLC-separated venom proteins was performed to characterize the dimeric disintegrins expressed in the venom proteome. In addition to previously reported EO4 and EO5 heterodimers, a novel dimeric disintegrin containing RGD- and KGD-bearing subunits was identified. However, a WGD-containing polypeptide encoded by clone Eo1-1 was not detected in the venom, suggesting the occurrence of larger genomic than proteomic diversity, which could represent part of a non-venom-secreted reservoir of disintegrin that may eventually acquire physiological relevance for the snake upon changes of ecological niches and prey habits. On the other hand, the realization of the existence of two distinct messengers coding for the short disintegrin ocellatusin reveals key events of the evolutionary emergence of the short disintegrin ocellatusin from a short-coding dimeric disintegrin precursor by two nucleotide mutations.









Similar content being viewed by others
References
Bilgrami S, Tomar S, Yadav S, Kaur P, Kumar J, Jabeen T, Sharma S, Sinhg TP (2004) Crystal structure of schistatin, a disintegrin homodimer from saw-scaled viper (Echis carinatus) at 2.5 Å resolution. J Mol Biol 341:829–837
Bilgrami S, Yadav S, Sharma S, Perbandt M, Betzel C, Singh TP (2005) Crystal structure of the disintegrin heterodimer from saw-scaled viper (Echis carinatus) at 1.9 Å resolution. Biochemistry 44:11058–11066
Calvete JJ (2005) Structure-function correlations of snake venom disintegrins. Curr Pharm Des 11:829–835
Calvete JJ, Moreno-Murciano MP, Sanz L, Jürgens M, Schrader M, Raida M, Benjamin DC, Fox JW (2000a) The disulfide bond pattern of catrocollastatin C, a disintegrin/cysteine-rich protein isolated from Crotalus atrox venom. Protein Sci 9:1365–1373
Calvete JJ, Jürgens M, Marcinkiewicz C, Romero A, Schrader M, Niewiarowski S (2000b) Disulfide bond pattern and molecular modelling of the dimeric disintegrin EMF-10, a potent and selective integrin α5β1 antagonist from Eristocophis macmahoni venom. Biochem J 345:573–581
Calvete JJ, Fox JW, Agelan A, Niewiarowski S, Marcinkiewicz C (2002) The presence of the WGD motif in CC8 heterodimeric disintegrin increases its inhibitory effect on αIIbβ3, αVβ3, and α5β1 integrins. Biochemistry 41:2014–2021
Calvete JJ, Moreno-Murciano MP, Theakston RDG, Kisiel DG, Marcinkiewicz C (2003) Snake venom disintegrins:novel dimeric disintegrins and structural diversification by disulfide bond engineering. Biochem J 372:725–734
Calvete JJ, Marcinkiewicz C, Monleón D, Esteve V, Celda B, Juárez P, Sanz L (2005) Snake venom disintegrins: evolution of structure and function. Toxicon 45:1063–1074
Daltry JC, Wüster W, Thorpe RS (1996) Diet and snake venom evolution. Nature 379:537–540
Francischetti IMB, My-Pham V, Harrison J, Garfield MK, Ribeiro JMC (2004) Bitis gabonica (Gaboon viper) snake venom gland:toward a catalog for the full-length transcripts (cDNA) and proteins. Gene 337:55–69
Fry BG (2005) From genome to “venome”: molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res 15:403–420
Fry BG, Wüster W (2004) Assembling an arsenal: origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol Biol Evol 21:870–883
Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SFR, Kuruppu S, Fung K, Hedges SB, Richardson MK, Hodgson WC, Ignjatovic V, Summerhayes R, Kochva E (2005) Early evolution of the venom system in lizards and snakes. Nature 439:584–588
Juárez P, Sanz L, Calvete JJ (2004) Snake venomics: characterization of protein families in Sistrurus barbouri venom by cysteine mapping, Nterminal sequencing, and tandem mass spectrometry analysis. Proteomics 4:327–338
Juárez P, Wagstaff SC, Oliver J, Sanz L, Harrison RA, Calvete JJ (2006) Molecular cloning of disintegrin-like transcript BA-5A from a Bitis arietans venom gland cDNA library: a putative intermediate in the evolution of the long-chain disintegrin bitistatin. J Mol Evol. Companion paper 10.1007/s00239-006-0268-z
Kini R, Evans HJ (1992) Structural domains in venom proteins: evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor. Toxicon 30:265–293
Kochva E (1987) The origin of snakes and evolution of the venom apparatus. Toxicon 25:65–106
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) Mega2: Molecular Evolutionary Genetics Analysis software. Bioinformatics 17:1244–1245
Ménez A (2002) Perspectives in molecular toxinology. John Wiley & Sons, Chichester, UK
Monleón D, Esteve V, Kovacs H, Calvete JJ, Celda B (2005) Conformation and concerted dynamics of the integrin-binding site and the C-terminal region of echistatin revealed by homonuclear NMR. Biochem J 387:57–66
Moreno-Murciano MP, Monleón D, Marcinkiewicz C, Calvete JJ, Celda B (2003) NMR solution structure of the non-RGD disintegrin obtustatin. J Mol Biol 329:135–145
Moura-Da-Silva AM, Theakston RDG, Crampton JM (1996) Evolution of disintegrin cysteine-rich and mammalian matrix-degrading metalloproteinases:gene duplication and divergence of a common ancestor rather than convergent evolution. J Mol Evol 43:263–269
Nikai T, Taniguchi K, Komori Y, Masuda K, Fox JW, Sugihara H (2000) Primary structure and functional characterization of bilitoxin-1, a novel dimeric P-II snake venom metalloproteinase from Agkistrodon bilineatus venom. Arch Biochem Biophys 378:6–15
Okuda D, Koike H, Morita T (2002) A new gene structure of the disintegrin family: a subunit of dimeric disintegrin has a short coding region. Biochemistry 41:14248–14254
Paine MJ, Desmond HP, Theakston RD, Crampton JM (1992) Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloprotease, jararhagin, from Bothrops jararaca venom. Insights into the disintegrin gene family. J Biol Chem 267:22869–22876
Sanz L, Bazaa A, Marrakchi N, Pérez A, Chenik M, Bel Lasfer Z, El Ayeb M, Calvete JJ (2006) Molecular cloning of disintegrins from Cerastes vipera and Macrovipera lebetina transmediterranea venom gland cDNA libraries. Insight into the evolution of the snake venom’s integrin inhibition system. Biochem J 395:385–392
Smith JB, Theakston RDG, Coelho ALJ, Barja-Fidalgo C, Calvete JJ, Marcinkiewicz C (2002) Characterization of a monomeric disintegrin, ocellatusin, present in the venom of the Nigerian carpet viper, Echis ocellatus. FEBS Lett 512:111–115
Scarborough RM, Rose JW, Hsu MA, Phillips DR, Fried VA, Campbell AM, Nannizzi L, Charo IF (2001) Barbourin, a GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem 266:9359–9362
Tani A, Ogawa T, Nose T, Nikandrov NN, Deshimaru M, Chijiwa T, Chang CC, Fukumaki Y, Ohno M (2002) Characterization, primary structure and molecular evolution of anticoagulant protein from Agkistrodon actus venom. Toxicon 40:803–813
Vidal N (2002) Colubroid systematics: evidence for an early appearance of the venom apparatus followed by extensive evolutionary tinkering. J Toxicol Toxin Rev 21:21–41
Acknowledgments
This work was financed by Grant BFU2004-01432 from the Ministerio de Educación y Ciencia, Madrid, Spain (to J.J.C.). P.J. and L.S. are recipients of a predoctoral fellowship (FPI; formación de personal investigador) from the Spanish Ministerio de Educación y Ciencia and a postdoctoral I3P contract, respectively. R.A.H. and S.C.W. were funded by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Reviewing Editor: Dr. Bryan Grieg Fry]
Rights and permissions
About this article
Cite this article
Juárez, P., Wagstaff, S.C., Sanz, L. et al. Molecular Cloning of Echis ocellatus Disintegrins Reveals Non-Venom-Secreted Proteins and a Pathway for the Evolution of Ocellatusin. J Mol Evol 63, 183–193 (2006). https://doi.org/10.1007/s00239-005-0269-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-005-0269-y