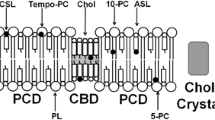
Abstract
The most unique feature of the eye lens fiber-cell plasma membrane is its extremely high cholesterol content. Cholesterol saturates the bulk phospholipid bilayer and induces formation of immiscible cholesterol bilayer domains (CBDs) within the membrane. Our results (based on EPR spin-labeling experiments with lens-lipid membranes), along with a literature search, have allowed us to identify the significant functions of cholesterol specific to the fiber-cell plasma membrane, which are manifest through cholesterol–membrane interactions. The crucial role is played by the CBD. The presence of the CBD ensures that the surrounding phospholipid bilayer is saturated with cholesterol. The saturating cholesterol content in fiber-cell membranes keeps the bulk physical properties of lens-lipid membranes consistent and independent of changes in phospholipid composition. Thus, the CBD helps to maintain lens-membrane homeostasis when the membrane phospholipid composition changes significantly. The CBD raises the barrier for oxygen transport across the fiber-cell membrane, which should help to maintain a low oxygen concentration in the lens interior. It is hypothesized that the appearance of the CBD in the fiber-cell membrane is controlled by the phospholipid composition of the membrane. Saturation with cholesterol smoothes the phospholipid-bilayer surface, which should decrease light scattering and help to maintain lens transparency. Other functions of cholesterol include formation of hydrophobic and rigidity barriers across the bulk phospholipid-cholesterol domain and formation of hydrophobic channels in the central region of the membrane for transport of small, nonpolar molecules parallel to the membrane surface. In this review, we provide data supporting these hypotheses.










Similar content being viewed by others
References
Altenbach C, Greenhalgh DA, Khorana HG, Hubbell WL (1994) A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci USA 91:1667–1671
Ashikawa I, Yin J-J, Subczynski WK, Kouyama T, Hyde JS, Kusumi A (1994) Molecular organization and dynamics in bacteriorhodopsin-rich reconstituted membranes: discrimination of lipid environments by the oxygen transport parameter using a pulse ESR spin-labeling technique. Biochemistry 33:4947–4952
Bach D, Wachtel E (2003) Phospholipid/cholesterol model membranes: formation of cholesterol crystallites. Biochim Biophys Acta 1610:187–197
Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP (2004) Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res 78:917–924
Bassnett S, McNulty R (2003) The effect of elevated intraocular oxygen on organelle degradation in the embryonic chicken lens. J Exp Biol 206:4353–4361
Beebe DC (2003) The lens. In: Kaufman PL (ed) Physiology of the eye. Mosby-Year Book, St. Louis, pp 117–158
Beebe DC (2008) Maintaining transparency: a review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol 19:125–133
Beebe DC, Holekamp NM, Siegfried C, Shui YB (2011) Vitreoretinal influences on lens function and cataract. Phil Trans R Soc Lond B Biol Sci 366:1293–1300
Bettelheim FA, Paunovic M (1979) Light scattering of normal human lens I. Application of random density and orientation fluctuation theory. Biophys J 26:85–99
Biswas SK, Lo WK (2007) Gap junctions contain different amounts of cholesterol which undergo unique sequestering processes during fiber cell differentiation in the embryonic chicken lens. Mol Vis 13:345–359
Biswas SK, Jiang JX, Lo WK (2009) Gap junction remodeling associated with cholesterol redistribution during fiber cell maturation in the adult chicken lens. Mol Vis 15:1492–1508
Biswas SK, Lee JE, Brako L, Jiang JX, Lo WK (2010) Gap junctions are selectively associated with interlocking ball-and-sockets but not protrusions in the lens. Mol Vis 16:2328–2341
Borchman D, Yappert MC (2010) Lipids and the ocular lens. J Lipid Res 51:2473–2488
Borchman D, Delamere NA, Cauley LA, Paterson CA (1989) Studies on the distribution of cholesterol, phospholipid and protein in the human and bovine lens. Lens Eye Exp Res 6:703–724
Borchman D, Lamba OP, Yappert MC (1993) Structural characterization of lipid membranes from clear and cataractous human lenses. Exp Eye Res 57:199–208
Borchman D, Byrdwell WC, Yappert MC (1994) Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest Ophthalmol Vis Sci 35:3938–3942
Borchman D, Cenedella RJ, Lamba OP (1996) Role of cholesterol in the structural order of lens membrane lipids. Exp Eye Res 62:191–197
Borchman D, Tang D, Yappert MC (1999) Lipid composition, membrane structure relationships in lens and muscle sarcoplasmic reticulum membranes. Biospectroscopy 5:151–167
Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D, Li L (2000) Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest Ophthalmol Vis Sci 41:3061–3073
Borchman D, Yappert MC, Afzal M (2004) Lens lipids and maximum lifespan. Exp Eye Res 79:761–768
Borochov N, Wachtel EJ, Bach D (1995) Phase behavior of mixtures of cholesterol and saturated phosphatidylglycerols. Chem Phys Lipids 76:85–92
Briggs D, Rodenhauser JH (1973) Distribution and consumption of oxygen in the vitreous body of cats. In: Kessler M (ed) Oxygen supply: theoretical and practical aspects of oxygen supply and microcirculation of tissue. University Park Press, Baltimore, pp 265–269
Broekhuyse RM (1973) Membrane lipids and proteins in aging lens and cataract. Ciba Found Symp 19:135–149
Broekhuyse RM, Kuhlmann ED (1974) Lens membranes 1. Composition of urea-treated plasma membranes from calf lens. Exp Eye Res 19:297–302
Broekhuyse RM, Kuhlmann ED (1978) Lens membranes. IV. Preparative isolation and characterization of membranes and various membrane proteins from calf lens. Exp Eye Res 26:305–320
Cenedella RJ (1996) Cholesterol and cataracts. Surv Ophthalmol 40:320–337
Cheetham JJ, Wachtel E, Bach D, Epand RM (1989) Role of the stereochemistry of the hydroxyl group of cholesterol and the formation of nonbilayer structures in phosphatidylethanolamines. Biochemistry 28:8928–8934
Chung CP, Hsu SY, Wu WC (2001) Cataract formation after pars plana vitrectomy. Kaohsiung J Med Sci 17:84–89
de Vries AC, Cohen LH (1993) Different effects of the hypolipidemic drugs pravastatin and lovastatin on the cholesterol biosynthesis of the human ocular lens in organ culture and on the cholesterol content of the rat lens in vivo. Biochim Biophys Acta 1167:63–69
Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, Truscott RJ (2008) Human lens lipids differ markedly from those of commonly used experimental animals. Biochim Biophys Acta 1781:288–298
Deeley JM, Hankin JA, Friedrich MG, Murphy RC, Truscott RJ, Mitchell TW, Blanksby SJ (2010) Sphingolipid distribution changes with age in the human lens. J Lipid Res 51:2753–2760
Eaton JW (1991) Is the lens canned? Free Radic Biol Med 11:207–213
Epand RM (2003) Cholesterol in bilayers of sphingomyelin or dihydrosphingomyelin at concentrations found in ocular lens membranes. Biophys J 84:3102–3110
Epand RM (2005) Role of membrane lipids in modulating the activity of membrane-bound enzymes. In: Yeagle PL (ed) The structure of biological membrane. CRC Press, Boca Raton, pp 499–509
Epand RM, Bain AD, Sayer BG, Bach D, Wachtel E (2002) Properties of mixtures of cholesterol with phosphatidylcholine or with phosphatidylserine studied by 13C magic angle spinning nuclear magnetic resonance. Biophys J 83:2053–2063
Estrada R, Puppato A, Borchman D, Yappert MC (2010) Reevaluation of the phospholipid composition in membranes of adult human lenses by 31P NMR and MALDI MS. Biochim Biophys Acta 1798:303–311
Fitch CL, Swedberg SH, Livesey JC (2000) Measurement and manipulation of the partial pressure of oxygen in the rat anterior chamber. Curr Eye Res 20:121–126
Fleschner CR, Cenedella RJ (1991) Lipid composition of lens plasma membrane fractions enriched in fiber junctions. J Lipid Res 32:45–53
Freel CD, Gilliland KO, Mekeel HE, Giblin FJ, Costello MJ (2003) Ultrastructural characterization and Fourier analysis of fiber cell cytoplasm in the hyperbaric oxygen treated guinea pig lens opacification model. Exp Eye Res 76:405–415
Friedrich MG, Truscott RJ (2009) Membrane association of proteins in the aging human lens: profound changes take place in the fifth decade of life. Invest Ophthalmol Vis Sci 50:4786–4793
Harding JJ (1991) Cataract, biochemistry, epidemiology and pharmacology. Chapman and Hall, London
Harocopos GJ, Shui YB, McKinnon M, Holekamp NM, Gordon MO, Beebe DC (2004) Importance of vitreous liquefaction in age-related cataract. Invest Ophthalmol Vis Sci 45:77–85
Helbig H, Hinz JP, Kellner U, Foerster MH (1993) Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol 2:161–164
Hsuan JD, Brown NA, Bron AJ, Patel CK, Rosen PH (2001) Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg 27:437–444
Huang J, Buboltz JT, Feigenson GW (1999) Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim Biophys Acta 1417:89–100
Huang L, Grami V, Marrero Y, Tang D, Yappert MC, Rasi V, Borchman D (2005) Human lens phospholipid changes with age and cataract. Invest Ophthalmol Vis Sci 46:1682–1689
Huang L, Estrada R, Yappert MC, Borchman D (2006) Oxidation-induced changes in human lens epithelial cells. 1. Phospholipids. Free Radic Biol Med 41:1425–1432
Huang L, Yappert MC, Jumblatt MM, Borchman D (2008) Hyperoxia and thyroxine treatment and the relationships between reactive oxygen species generation, mitochondrial membrane potential, and cardiolipin in human lens epithelial cell cultures. Curr Eye Res 33:575–586
Hubbell WL, McConnell HM (1968) Spin-label studies of the excitable membranes of nerve and muscle. Proc Natl Acad Sci USA 61:12–16
Jacob RF, Cenedella RJ, Mason RP (1999) Direct evidence for immiscible cholesterol domains in human ocular lens fiber cell plasma membranes. J Biol Chem 274:31613–31618
Jacob RF, Cenedella RJ, Mason RP (2001) Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. J Biol Chem 276:13573–13578
Jacobi KW, Driest J (1966) Oxygen determinations in the vitreous body of the living eye [in German]. Ber Zusammenkunft Dtsch Ophthalmol Ges 67:193–198
Jost PC, Griffith OH, Capaldi RA, Vanderkooi G (1973) Evidence for boundary lipid in membranes. Proc Natl Acad Sci USA 70:480–484
Kawasaki K, Yin J–J, Subczynski WK, Hyde JS, Kusumi A (2001) Pulse EPR detection of lipid exchange between protein rich raft and bulk domains in the membrane: methodology development and its application to studies of influenza viral membrane. Biophys J 80:738–748
Kirby TJ (1967) Cataracts produced by triparanol (MER-29). Trans Am Ophthalmol Soc 65:494–543
Knoll W, Schmidt G, Ibel K, Sackmann E (1985) Small-angle neutron scattering study of lateral phase separation in dimyristoylphosphatidylcholine-cholesterol mixed membranes. Biochemistry 24:5240–5246
Kusumi A, Subczynski WK, Hyde JS (1982) Oxygen transport parameter in membranes as deduced by saturation recovery measurements of spin-lattice relaxation times of spin labels. Proc Natl Acad Sci USA 79:1854–1858
Kusumi A, Subczynski WK, Pasenkiewicz-Gierula M, Hyde JS, Merkle H (1986) Spin-label studies on phosphatidylcholine-cholesterol membranes: effects of alkyl chain length and unsaturation in the fluid phase. Biochim Biophys Acta 854:307–317
Li LK, So L (1987) Age dependent lipid and protein changes in individual bovine lenses. Curr Eye Res 6:599–605
Li LK, So L, Spector A (1985) Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J Lipid Res 26:600–609
Li LK, So L, Spector A (1987) Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim Biophys Acta 917:112–120
Mainali L, Feix JB, Hyde JS, Subczynski WK (2011a) Membrane fluidity profiles as deduced by saturation-recovery EPR measurements of spin-lattice relaxation times of spin labels. J Magn Reson 212:418–425
Mainali L, Raguz M, Camenisch TG, Hyde JS, Subczynski WK (2011b) Spin-label saturation-recovery EPR at W-band: applications to eye lens lipid membranes. J Magn Reson 212:86–94
Mainali L, Raguz M, Subczynski WK (2011c). Phases and domains in sphingomyelin-cholesterol membranes: structure and properties using EPR spin-labeling methods. Eur Biophys J. doi:10.1007/s00249-011-0766-4
Mainali L, Raguz M, Subczynski WK (2011d) Phase-separation and domain-formation in cholesterol-sphingomyelin mixture: pulse-EPR oxygen probing. Biophys J 101:837–846
Marsh D (1981) Electron spin resonance: spin labels. In: Grell E (ed) Membrane spectroscopy. Springer-Verlag, Berlin, pp 51–142
Mason RP, Tulenko TN, Jacob RF (2003) Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim Biophys Acta 1610:198–207
McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJ, Bassnett S (2004) Regulation of tissue oxygen levels in the mammalian lens. J Physiol 559:883–898
Moffat BA, Landman KA, Truscott RJ, Sweeney MH, Pope JM (1999) Age-related changes in the kinetics of water transport in normal human lenses. Exp Eye Res 69:663–669
Mosley ST, Kalinowski SS, Schafer BL, Tanaka RD (1989) Tissue-selective acute effects of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase on cholesterol biosynthesis in lens. J Lipid Res 30:1411–1420
Ormerod LD, Edelstein MA, Schmidt GJ, Juarez RS, Finegold SM, Smith RE (1987) The intraocular environment and experimental anaerobic bacterial endophthalmitis. Arch Ophthalmol 105:1571–1575
Palmquist BM, Philipson B, Barr PO (1984) Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol 68:113–117
Plesnar E, Subczynski WK, Pasenkiewicz-Gierula, M (2011). Saturation with cholesterol increases vertical order and smoothes the surface of the phosphatidylcholine bilayer: a molecular simulation study. Biochim Biophys Acta. doi:10.1016/j.bbamem.2011.10.023
Paterson CA, Zeng J, Husseini Z, Borchman D, Delamere NA, Garland D, Jimenez-Asensio J (1997) Calcium ATPase activity and membrane structure in clear and cataractous human lenses. Curr Eye Res 16:333–338
Peterson CA, Delamere NA (1992) The lens. In: Hart WM Jr (ed) Physiology of the eye. Mosby-Year Book, St. Louis, pp 348–390
Rafferty NS (1985) Lens morphology. In: Maisel H (ed) The ocular lens: structure, function and pathology. Marcel Dekker, New York, pp 1–60
Raguz M, Widomska J, Dillon J, Gaillard ER, Subczynski WK (2008) Characterization of lipid domains in reconstituted porcine lens membranes using EPR spin-labeling approaches. Biochim Biophys Acta 1778:1079–1090
Raguz M, Widomska J, Dillon J, Gaillard ER, Subczynski WK (2009) Physical properties of the lipid bilayer membrane made of cortical and nuclear bovine lens lipids: EPR spin-labeling studies. Biochim Biophys Acta 1788:2380–2388
Raguz M, Mainali L, Widomska J, Subczynski WK (2011a) The immiscible cholesterol bilayer domain exists as an integral part of phospholipid bilayer membranes. Biochim Biophys Acta 1808:1072–1080
Raguz M, Mainali L, Widomska J, Subczynski WK (2011b). Using spin-label electron paramagnetic resonance (EPR) to discriminate and characterize the cholesterol bilayer domain. Chem Phys Lipids 164:819–829
Rouser G, Solomon RD (1969) Changes in phospholipid composition of human aorta with age. Lipids 4:232–234
Rouser G, Simon G, Kritchevsky G (1969) Species variations in phospholipid class distribution of organs. I. Kidney, liver and spleen. Lipids 4:599–606
Rouser G, Yamamoto A, Kritchevsky G (1971) Cellular membranes. Structure and regulation of lipid class composition species differences, changes with age, and variations in some pathological states. Arch Intern Med 127:1105–1121
Roy D, Rosenfeld L, Spector A (1982) Lens plasma membrane: isolation and biochemical characterization. Exp Eye Res 35:113–129
Rujoi M, Jin J, Borchman D, Tang D, Yappert MC (2003) Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Invest Ophthalmol Vis Sci 44:1634–1642
Rujoi M, Estrada R, Yappert MC (2004) In situ MALDI-TOF MS regional analysis of neutral phospholipids in lens tissue. Anal Chem 76:1657–1663
Ryba NJ, Horvath LI, Watts A, Marsh D (1987) Molecular exchange at the lipid–rhodopsin interface: spin-label electron spin resonance studies of rhodopsin-dimyristoylphosphatidylcholine recombinants. Biochemistry 26:3234–3240
Shui YB, Fu JJ, Garcia C, Dattilo LK, Rajagopal R, McMillan S, Mak G, Holekamp NM, Lewis A, Beebe DC (2006) Oxygen distribution in the rabbit eye and oxygen consumption by the lens. Invest Ophthalmol Vis Sci 47:1571–1580
Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, Beebe DC (2009) The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol 127:475–482
Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC (2010) Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci 51:5731–5738
Subczynski WK, Hyde JS (1998) Membranes. Barriers or pathways for oxygen transport. Adv Exp Med Biol 454:399–408
Subczynski WK, Hyde JS, Kusumi A (1989) Oxygen permeability of phosphatidylcholine-cholesterol membranes. Proc Natl Acad Sci USA 86:4474–4478
Subczynski WK, Hyde JS, Kusumi A (1991) Effect of alkyl chain unsaturation and cholesterol intercalation on oxygen transport in membranes: a pulse ESR spin labeling study. Biochemistry 30:8578–8590
Subczynski WK, Renk GE, Crouch RK, Hyde JS, Kusumi A (1992) Oxygen diffusion-concentration product in rhodopsin as observed by a pulse ESR spin labeling method. Biophys J 63:573–577
Subczynski WK, Wisniewska A, Yin J-J, Hyde JS, Kusumi A (1994) Hydrophobic barriers of lipid bilayer membranes formed by reduction of water penetration by alkyl chain unsaturation and cholesterol. Biochemistry 33:7670–7681
Subczynski WK, Lewis RN, McElhaney RN, Hodges RS, Hyde JS, Kusumi A (1998) Molecular organization and dynamics of 1-palmitoyl-2-oleoylphosphatidylcholine bilayers containing a transmembrane alpha-helical peptide. Biochemistry 37:3156–3164
Subczynski WK, Pasenkiewicz-Gierula M, McElhaney RN, Hyde JS, Kusumi A (2003) Molecular dynamics of 1-palmitoyl-2-oleoylphosphatidylcholine membranes containing transmembrane alpha-helical peptides with alternating leucine and alanine residues. Biochemistry 42:3939–3948
Subczynski WK, Widomska J, Wisniewska A, Kusumi A (2007a) Saturation-recovery electron paramagnetic resonance discrimination by oxygen transport (DOT) method for characterizing membrane domains. Methods Mol Biol 398:143–157
Subczynski WK, Wisniewska A, Hyde JS, Kusumi A (2007b) Three-dimensional dynamic structure of the liquid-ordered domain as examined by a pulse-EPR oxygen probing. Biophys J 92:1573–1584
Subczynski WK, Raguz M, Widomska J (2010) Studying lipid organization in biological membranes using liposomes and EPR spin labeling. Methods Mol Biol 606:247–269
Sweeney MH, Truscott RJ (1998) An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res 67:587–595
Truscott RJ (2000) Age-related nuclear cataract: a lens transport problem. Ophthalmic Res 32:185–194
Tulenko TN, Chen M, Mason PE, Mason RP (1998) Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J Lipid Res 39:947–956
Wachtel EJ, Borochov N, Bach D (1991) The effect of protons or calcium ions on the phase behavior of phosphatidylserine-cholesterol mixtures. Biochim Biophys Acta 1066:63–69
Widomska J, Raguz M, Dillon J, Gaillard ER, Subczynski WK (2007a) Physical properties of the lipid bilayer membrane made of calf lens lipids: EPR spin labeling studies. Biochim Biophys Acta 1768:1454–1465
Widomska J, Raguz M, Subczynski WK (2007b) Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim Biophys Acta 1768:2635–2645
Wisniewska A, Subczynski WK (2008) The liquid-ordered phase in sphingomyelin-cholesterol membranes as detected by the discrimination by oxygen transport (DOT) method. Cell Mol Biol Lett. 13:430–451
Yappert MC, Borchman D (2004) Sphingolipids in human lens membranes: an update on their composition and possible biological implications. Chem Phys Lipids 129:1–20
Yappert MC, Rujoi M, Borchman D, Vorobyov I, Estrada R (2003) Glycero- versus sphingo-phospholipids: correlations with human and non-human mammalian lens growth. Exp Eye Res 76:725–734
Zelenka PS (1984) Lens lipids. Curr Eye Res 3:1337–1359
Acknowledgements
This work was supported by grants EY015526, TW008052, EB002052 and EB001980 of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subczynski, W.K., Raguz, M., Widomska, J. et al. Functions of Cholesterol and the Cholesterol Bilayer Domain Specific to the Fiber-Cell Plasma Membrane of the Eye Lens. J Membrane Biol 245, 51–68 (2012). https://doi.org/10.1007/s00232-011-9412-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-011-9412-4