Abstract
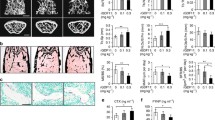
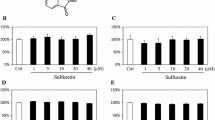
Growth differentiation factor 11 (GDF11) is a member of the transforming growth factor-β superfamily. Recent studies confirmed that GDF11 plays an important role in regulating the regeneration of brain, skeletal muscle, and heart during aging; however, its role in bone metabolism remains unclear. Thus, the aim of this study was to determine the effects of GDF11 on bone metabolism, including bone formation and bone resorption, both in vitro and in vivo. Our results showed that GDF11 inhibited osteoblastic differentiation of bone marrow mesenchymal stem cells in vitro. Mechanistically, GDF11 repressed Runx2 expression by inducing SMAD2/3 phosphorylation during osteoblast differentiation. Moreover, intraperitoneal injection of GDF11 inhibited bone formation and accelerated age-related bone loss in mice. Our results also showed that GDF11 had no effect on osteoclast differentiation or bone resorption both in vitro and in vivo. These results provide a further rationale for the therapeutic targeting of GDF11 for the treatment of age-related osteoporosis.





Similar content being viewed by others
References
Loffredo FS, Steinhauser ML, Jay SM et al (2013) Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153:828–839
Katsimpardi L, Litterman NK, Schein PA et al (2014) Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344:630–634
Sinha M, Jang YC, Oh J et al (2014) Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344:649–652
Smith SC, Zhang X, Zhang X et al (2015) GDF11 does not rescue aging-related pathological hypertrophy. Circ Res 117:926–932
Mendelsohn AR, Larrick JW (2014) Systemic factors mediate reversible age-associated brain dysfunction. Rejuvenation Res 17:525–528
Egerman MA, Cadena SM, Gilbert JA et al (2015) GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab 22:164–174
Raisz L (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115:3318–3325
Ettinger B, Black DM, Nevitt MC et al (1992) Contribution of vertebral deformities to chronic pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 7:449–456
Cauley JA, Thompson DE, Ensrud KC et al (2000) Risk of mortality following clinical fractures. Osteoporos Int 11:556–561
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. 11:219–227
Zhang Y, Shao J, Wang Z et al (2015) Growth differentiation factor 11 is a protective factor for osteoblast genesis by targeting PPAR gamma. Gene 557:209–214
Shoback D (2007) Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab 92:747–753
Li H, Xie H, Liu W et al (2009) A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest 119:3666–3677
Shim JH, Greenblatt MB, Zou W et al (2013) Schnurri-3 regulates ERK downstream of WNT signaling in osteoblasts. J Clin Invest. 123:4010–4022
Li Z, Kawasumi M, Zhao B et al (2010) Transgenic over-expression of growth differentiation factor 11 propeptide in skeleton results in transformation of the seventh cervical vertebra into a thoracic vertebra. Mol Reprod Dev. 77:990–997
Li CJ, Cheng P, Liang MK et al (2015) MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest 125:1509–1522
Liu Y, Berendsen AD, Jia S et al (2012) Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 122:3101–3113
Cao Y, Gomes SA, Rangel EB et al (2015) S-nitrosoglutathione reductase-dependent PPARγ denitrosylation participates in MSC-derived adipogenesis and osteogenesis. J Clin Invest 125:1679–1691
Nishikawa K, Nakashima T, Takeda S et al (2010) Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest 120:3455–3465
Iyer S, Ambrogini E, Bartell SM et al (2013) FOXOs attenuate bone formation by suppressing Wnt signaling. J Clin Invest. 123:3409–3419
Wu MY, Hill CS (2009) TGF—beta superfamily signaling in embryonic development and homeostasis. Dev Cell 16:329–343
Wu JY, Aarnisalo P, Bastepe M et al (2011) Gsα enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 121:3492–3504
Sartori R, Milan G, Patron M et al (2009) Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 296:C1248–C1257
Kang JS, Alliston T, Delston R et al (2005) Repression of Runx2 function by TGF -beta through recruitment of class II histone deacetylases by Smad3. EMBO J 24:2543–2555
Chen G, Deng C, Li YP et al (2012) TGF- β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 8:272–288
Hamrick MW (2003) Increased bone mineral density in the of GDF8 knockout mice. Anat Rec A DiscovMol Cell Evol Biol 272:388–391
Hamrick MW, Shi X, Zhang W et al (2007) Loss of myostatin (gdf8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 40:1544–1553
Hamrick MW, McPherron AC, Lovejoy CO (2002) Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif Tissue Int 71:63–68
McPherron AC, Lawler AM, Lee SJ (1999) Regulation of anterior/posterior patterning of the axial skeleton by growth differentiation factor 11. Nat Genet 22:260–264
Hamrick MW, Arounleut P, Kellum E et al (2010) Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J Trauma 69:579–583
Acknowledgments
This work was supported by Grant No. 81520108008 from the Major International (Regional) Joint Research Project of China National Natural Scientific Foundation (NSFC), Grant No. 81125006 from the Distinguished Young Scientists of China National Natural Scientific Foundation, Grant No. U1301222 from the NSFC-Guangdong Joint Project, and Grant No. 81570806 from the China National Natural Scientific Foundation.
Author Contributions
Study design: XHL; Study conduct: QL, MLT, CJL, ZL, TL, and XHL; Data collection: QL, MLT, CJL, and ZL; Data analysis: XHL, QL, MLT, and CJL; Data interpretation: XHL, QL, MLT, CJL, and LZ; Drafting manuscript: XHL, QL, MLT, and CJL; Revising manuscript content: XHL and QL; Approval of the final manuscript: QL, MLT, CJL, ZL, TL and XHL; XHL and QL are primarily responsible for integrity of the data analysis, and all authors take responsibility for and attest to the integrity of the data analysis. XHL is responsible for transmitting the editors’ comments to the other authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Xiang-Hang Luo, Qiong Lu, Man-Li Tu, Chang-Jun Li, Li Zhang, Tie-Jian Jiang, and Tang Liu declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Mouse cell line and mice were used in this study. All animal experiments were approved by the Animal Care and Use Committee of the Laboratory Animal Research Center at Xiangya Medical School of Central South University (Changsha, Hunan, China). Strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals. No human specimen was used in this study.
Rights and permissions
About this article
Cite this article
Lu, Q., Tu, ML., Li, CJ. et al. GDF11 Inhibits Bone Formation by Activating Smad2/3 in Bone Marrow Mesenchymal Stem Cells. Calcif Tissue Int 99, 500–509 (2016). https://doi.org/10.1007/s00223-016-0173-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0173-z