Abstract
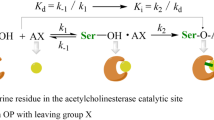
Organophosphates are a group of pesticides and chemical warfare nerve agents that inhibit acetylcholinesterase, the enzyme responsible for hydrolysis of the excitatory neurotransmitter acetylcholine. Numerous structural variants exist for this chemical class, and data regarding their toxicity can be difficult to obtain in a timely fashion. At the same time, their use as pesticides and military weapons is widespread, which presents a major concern and challenge in evaluating human toxicity. To address this concern, a quantitative structure–activity relationship (QSAR) was developed to predict pentavalent organophosphate oxon human acetylcholinesterase bimolecular rate constants. A database of 278 three-dimensional structures and their bimolecular rates was developed from 15 peer-reviewed publications. A database of simplified molecular input line entry notations and their respective acetylcholinesterase bimolecular rate constants are listed in Supplementary Material, Table I. The database was quite diverse, spanning 7 log units of activity. In order to describe their structure, 675 molecular descriptors were calculated using AMPAC 8.0 and CODESSA 2.7.10. Orthogonal projection to latent structures regression, bootstrap leave-random-many-out cross-validation and y-randomization were used to develop an externally validated consensus QSAR model. The domain of applicability was assessed by the William’s plot. Six external compounds were outside the warning leverage indicating potential model extrapolation. A number of compounds had residuals >2 or <−2, indicating potential outliers or activity cliffs. The results show that the HOMO–LUMO energy gap contributed most significantly to the binding affinity. A mean training R 2 of 0.80, a mean test set R 2 of 0.76 and a consensus external test set R 2 of 0.66 were achieved using the QSAR. The training and external test set RMSE values were found to be 0.76 and 0.88. The results suggest that this QSAR model can be used in physiologically based pharmacokinetic/pharmacodynamic models of organophosphate toxicity to determine the rate of acetylcholinesterase inhibition.






Similar content being viewed by others
References
Agabekyan RS, Berkhamov MK, Godovikov NN, Kabachnik MI, Ol’khovaya GG (1974) Synthesis and anticholinesterase properties of O-ethyl-S-(Beta-alkylsulfoxyethyl)methylthiophosphonates. Russ Chem Bull 23:1288–1290
Agabekyan RS, Berkhamov MK, Kuzamyshev VM, Muzykantova VN, Bekanov MKh, Kabachnik MI (1977) Reaction of O-ethyl-S-(Beta-alkylmercaptoethyl)benzylthiophosphonates and their iodomethylates with cholinesterases. Russ Chem Bull 26:1730–1734
Aldridge WN, Reiner E (1972) Enzyme inhibitors as substrates: interactions of Esterases with esters of organophosphorus and carbamic acids, vol 1. American Elsevier Publishing Co, New York, pp 1–328
Berkhamov MK, Zakharova LM, Grineva LG, Kuzamyshev VM, Ol’khovaya GG, Bekanov MK et al (1981) Synthesis and anticholinesterase activity of S-Beta-aryl-and S-Beta-Benzylmercaptoethyl esters of thioacids of phosphorus. Russ Chem Bull 30:658–663
Brestkin AP, Godovikov NN (1978) Combined inhibition of Cholinesterases by organophosphorus compounds. Russ Chem Rev 47:859–869
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 4:187–217
Consonni V, Ballabio D, Todeschini R (2009) Comments on the definition of the Q2 parameter for QSAR validation. J Chem Inf Model 49:1669–1678
Cronin MTD, Schultz TW (2003) Pitfalls in QSAR. J Mol Struct (Theochem) 622:39–51
Dudek AZ, Arodz T, Galvez J (2006) Computational methods in developing quantitative structure-activity relationships (QSAR): a review. Comb Chem High T Scr 9:213–228
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Eriksson L, Jaworska J, Worth AP, Cronin MTD, McDowell RM, Gramatica P (2003) Methods for reliability and uncertainty assessment and for the applicability evaluations of classification- and regression-based QSARs. Environ Health Perspect 111:1361–1375
Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87:245–254
Golbraikh A, Tropsha A (2002) Beware of q2! J Mol Graph Model 20:269–276
Gramatica P (2007) Principles of QSAR model validation: internal and external. QSAR Comb Sci 26:694–701
Grigoryan H, Schopfer LM, Peeples ES, Duysen EG, Grigoryan M, Thompson CM, Lockridge O (2009) Mass spectrometry identifies multiple organophosphorylated sites on tubulin. Toxicol Appl Pharm 240:149–158
Guha R, Van Drie JH (2008) Structure-activity landscape index: identifying and quantifying activity cliffs. J Chem Inf Model 48:646–658
Hewitt M, Cronin MTD, Madden JC, Rowe PH, Johnson C, Obi A, Enoch SJ (2007) Consensus QSAR models: do the benefits outweigh the complexity? J Chem Inf Model 47:1460–1468
Ivanciuc O (1997) CODESSA Version 2.13 for Windows. J Chem Inf Comput Sci 37:405–406
Jaworska J, Nikolova-Jeliazkova N, Aldenberg T (2005) QSAR applicability domain estimation by projection of the training set in descriptor space: a review. ATLA 33:445–459
Johnson SR (2008) The trouble with QSAR (or how I learned to stop worrying and embrace fallacy). J Chem Inf Model 48:25–26
Kabachnik MI, Brestkin AP, Godovikov NN, Michelson MJ, Rozengart EV, Rozengart VI (1970) Hydrophobic areas on the active surface of cholinesterases. Pharmacol Rev 22:355–388
Katritzky AR, Wang Y, Sild S, Tamm T, Karelson M (1998) QSPR studies on vapor pressure, aqueous solubility, and the prediction of water-air partition coefficients. J Chem Inf Comput Sci 38:720–725
Katritzky AR, Kuanar M, Fara DC, Karelson M, Acree WE, Solov’ev VP et al (2005) QSAR modeling of blood:air and tissue:air partition coefficients using theoretical descriptors. Bioorg Med Chem 13:6450–6463
Kuzamyshev VM, Berkhamov MK, Godovikov NN, Agabekyan RS, Kireeva EG, Pegova ZK et al (1977) Reaction of O-ethyl-S-(ß-alkylmercaptoethl)-cyclohexylthiophosphonates and their iodomethylates with choline esterases. Russ Chem Bull 26:1471–1476
Lee AH, Metcalf RL (1973) In vitro inhibition of acetylcholinesterase by O, O-dimethyl-S-aryl phosphorothioates. Pesticide Biochem Physiol 2:408–417
Maggiora GM (2006) On outliers and activity cliffs-Why QSAR often disappoints. J Chem Inf Model 46:1535
Makhaeva GF, Filonenko IV, Yankovskaya VL, Fomicheva SB, Malygin VV (1998) Comparative studies of O, O-dialkyl-O-chloromethylchloroformimino phosphates: interaction with neuropathy target esterase and acetylcholinesterase. Neurotoxicology 19:623–628
Makhaeva GF, Aksinenko AY, Sokolov VB, Serebryakova OG, Richardson RJ (2009) Synthesis of organophosphates with fluorine-containing leaving groups as serine esterase inhibitors with potential for Alzheimer disease therapeutics. Bioorgan Med Chem Lett 19:5528–5530
Mastryukova TA, Agabekyan RS, Uryupin AB, Kabachnik MI (1978) Synthesis and anticholinesterase properties of some S-benzhydryl esters of phosphorus monothio acids. Russ Chem Bull 26:2153–2157
Munro NB, Ambrose KR, Watson AP (1994) Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: implications for public protection. Environ Health Perspect 102:18–38
OECD Environment Health and Safety Publications. Series on Testing and Assessment No. 69 (2007) Guidance document on the validation of (Quantitative) structure-activity relationship [(Q)SAR] models. Organisation for Economic Co-Operation and Development, Paris
Ol’khovaya GG, Agabekyan RS, Berkhamov MK, Godovikov NN, Kabachnik MI (1976a) Synthesis and reaction of O-ethyl S-alkyl phenylthiophosphonates with cholinesterase. Russ Chem Bull 24:1719–1721
Ol’khovaya GG, Agabekyan RS, Berkhamov MK, Godovikov NN, Kabachnik MI (1976b) Synthesis and reaction of O, O-dibutyl S-alkyl thiophosphates with cholinesterase. Russ Chem Bull 24:1722–1724
Plyamovatyi AK, Vandyukova II, Shagidullin RR, Makhaeva GF, Malygin VV, Gorbunov SM (1997) Study of the relationship between spacial structure and anticholinesterse activity of O-phosphorylate oximes. Pharm Chem J 31:199–204
Raevskii OA, Chistiakov VV, Agabekian RS, Sapegin AM, Zefirov NS (1990) Formation of models of the interaction between organophosphate compound structure and their ability to inhibit cholinesterase. Bioorg Khim 16:1509–1522
Raveh L, Grunwald J, Marcus D, Papier Y, Cohen E, Ashani Y (1993) Human butyrylcholinesterase as a general prophylactic antidote for nerve agent toxicity. In vitro and in vivo quantitative characterization. Biochem Pharmacol 45:2465–2474
Ruark CD, Hack CE, Robinson PJ, Gearhart JM (2011) Quantitative structure-activity relationships for organophosphates binding to trypsin and chymotrypsin. J Toxicol Environ Health 74:1–18
Rucker C, Rucker G, Meringer M (2007) Y-randomization and its variants in QSPR/QSAR. J Chem Inf Model 47:2345–2357
Sadykov AS, Dalimov DN, Godovikov NN (1983) Phosphorylated derivatives of alkaloids and nitrogen-containing heterocycles-Cholinesterase inhibitors. Russian Chem Rev 52:918–930
Tropsha A (2010) Best practices for QSAR model development, validation, and exploitation. Mol Inf 29:476–488
Tropsha A, Gramatica P, Gombar VK (2003) The importance of being earnest: validation is the absolute essential for successful application and interpretation of QSPR models. QSAR Comb Sci 22:69–77
Trygg J, Wold S (2002) Orthogonal projections to latent structures (O-PLS). J Chemometr 16:119–128
Vale JA (2007) Nerve agents: why they are so toxic and can poisoning from these agents be treated? Toxicology 240:141–142
Volkova RI, Brestkin AP, Kochetova LM (1982) Stereospecificity of the active site of acylcholinesterases. Biochemistry 47:669–675
Votano JR, Parham M, Hall LH, Kier LB, Oloff S, Tropsha A, Xie Q, Tong W (2004) Three new consensus QSAR models for the prediction of Ames genotoxicity. Mutagenesis 19:365–377
Wang C, Murphy SD (1982) Kinetic analysis of species difference in acetylcholinesterase sensitivity to organophosphate insecticides. Toxicol Appl Pharmacol 66:409–419
Yang RS, Thomas RS, Gustafson DL, Campain J, Benjamin SA, Verhaar HJ et al (1998) Approaches to developing alternative and predictive toxicology based on PBPK/PD and QSAR modeling. Environ Health Perspect 106:1385–1393
Acknowledgments
This work was supported by the Defense Threat Reduction Agency—Joint Science and Technology Office, Basic and Supporting Sciences Division [2.G 806 08 AHB C].
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruark, C.D., Hack, C.E., Robinson, P.J. et al. Quantitative structure–activity relationships for organophosphates binding to acetylcholinesterase. Arch Toxicol 87, 281–289 (2013). https://doi.org/10.1007/s00204-012-0934-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-012-0934-z