Abstract
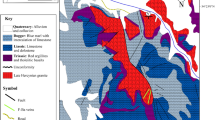
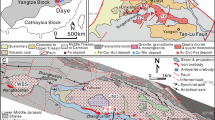
Fluorite from Mississippi Valley Type (MVT) deposits in the South Pennine Orefield, England, displays significantly different distributions of rare earths and yttrium (REY) compared to fluorite from similar MVT deposits in the North Pennine Orefield. Samples from the South Pennine Orefield display negative Ce and positive Gd and Y anomalies but lack any Eu anomaly, indicating that the REY were mobilized from relatively pure marine sedimentary carbonates. In marked contrast, fluorite from the North Pennine Orefield lacks any Ce and Gd anomalies but shows a pronounced positive Eu anomaly, suggesting that the REY were provided by different source rock(s), that the mineralizing hydrothermal fluid had experienced higher temperatures prior to fluorite precipitation, and that it was derived from deeper crustal levels in the north compared to the south. The isotopic composition of Sr in Blue John fluorite from the South Pennine Orefield suggests that Sr was mobilized from Lower Carboniferous (Tournaisian) limestones, whereas Pb isotopes suggest that in contrast to REY and Sr, Pb was derived from aluminosilicate rocks. Neither Nd nor Sr or Pb isotopes can be used to radiometrically date the formation of Blue John fluorite. All isotope systems studied indicate that the limestone host rock of this fluorite mineralization did not contribute to the trace element budget of the hydrothermal fluid. Our results show that different solutes in a natural water (hydrothermal fluid, groundwater, etc.) may be derived from different sources, and that the study of a small set of elements or isotope ratios may not provide full insight into the genesis or history of a mineralization or a hydrothermal fluid. Our data provide evidence for the uncoupling of Sr, Nd and Pb during fluid-rock interaction and fluid migration, and show that the use of plots such as 87Sr/86Sr vs. εNd. to learn about mixing relationships (as is commonly done in igneous geochemistry) is unreliable when applied to natural waters and their precipitates.










Similar content being viewed by others
References
Anderson G, Macqueen RW (1982) Ore deposit models 6: MVT type lead-zinc deposits. Geosci Can 9:108–117
Atkinson P (1983) A fluid inclusion and geochemical investigation of fluorite deposits of the Southern Pennine Orefield. PhD Thesis, Univ Leicester, 284 pp
Bau M (1996) Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib Mineral Petrol 123:323–333
Bau M, Möller P (1992) Rare earth element fractionation in metamorphogenic hydrothermal calcite, magnesite and siderite. Mineral Petrol 45:231–256
Bau M, Dulski P (1995) Comparative study of yttrium and rare-earth element behaviour in fluorine-rich hydrothermal fluids. Contrib Mineral Petrol 119:213–223
Bau M, Dulski P (1996) Distribution of yttrium and rare-earth elements in the Penge and Kuruman Iron-Formations, Transvaal Supergroup, South Africa. Precambrian Res 79:37–55
Bilal BA, Müller E (1992) Thermodynamic study of Ce4+/Ce3+ redox reaction in aqueous solutions at elevated temperatures. 1. Reduction potential of Ce4+ in perchloric acid solution. Z Naturforsch A: Phys Sci 47:231–246
Brannon JC, Podesk FA, Viets, JG, Leach DL, Goldhaber, M, Rowan EL (1991) Strontium isotope constraints on the origin of ore-forming fluids of the Viburnum Trend, southeast Missouri. Geochim Cosmochim Acta 55:1407–1419
Bruckschen P, Bruhn F, Veitzer J, Buhl D (1995) 87Sr/86Sr isotopic evolution of Lower Carboniferous seawater; Dinantian of western Europe. Sediment Geol 100:63–81
Burke WH, Denison RE, Hetherington EA, Koepnick RB, Nelson HF, Otto JB (1982) Variation of seawater 87Sr/86Sr throughout Phanerozoic time. Geol 10:516–519
Butcher NJD, Hedges JD (1987) Exploration and extraction of structurally and lithostratigraphically controlled fluorite deposits in Castleton-Bradwell area of Southern Pennine Orefield, England. Trans Inst Min Metall B96:149–155
Chesley JT, Halliday AN, Scrivener RC (1991) Samarium-Neodymium direct dating of fluorite mineralization. Science 252:949–951
Chesley TJ, Halliday AN, Kyser TK, Spry PG (1994) Direct dating of Mississippi Valley-type mineralization: use of Sm-Nd in fluorite. Econ Geol 89:1192–1199
Cherniak DJ, Zhang,XY, Wayne NK, Watson EB (2001) Sr, Y, and REE diffusion in fluorite. Chem Geol 181:99–111
Crocetti CA, Holland HD (1989) Sulfur-lead isotopic systems and the composition of fluid inclusions in galena from the Viburnum Trend, Missouri. Econ Geol 84:1296–2216
Davis G, Gledhill A, Hawkesworth C (1985) Upper crustal recycling in southern Britain: evidence from Nd and Sr isotopes. Earth Planet Sci Lett 75:1–12
Dill H, Dulski P, Möller P (1986) Fluorite mineralization and REE patterns in vein-type deposits from the N Bavarian Basement. Neues Jahrb Min Abh 154:141–151
Dulski P (1994) Interferences of oxide, hydroxide and chloride analyte species in the determination of rare earth elements in geological samples by inductively coupled plasma-mass spectrometry. Fresenius' J Anal Chem 350:194–203
Dulski P (2001) Reference materials for geochemical studies: new analytical data by ICP-MS and critical discussion of reference values. Geost Newsl, J Geostand Geoanal 25:87–125
Dunham KC (1983) Ore genesis in the English Pennines: a fluorite subtype. In: Kisvarsanyi G, Grant SK, Pratt WP, Koenig JW (eds) International conference on Mississippi Valley-type lead-zinc deposits. Proc Vol, Univ of Missouri-Rolla, pp 86–112
Eppinger, RG, Closs LG (1990) Variation of trace elements and rare earth elements in fluorite; a possible tool for exploration. Econ Geol 85:1896–1907
Ewbank G, Abbott, GD, Manning DAC (1993) An organic geochemical study of bitumens and their potential source rocks from the South Pennine Orefield, Central England. Org Geochem 20:579–598
Ewbank G, Manning DAC, Abbott, GD (1995) The relationship between bitumens and mineralization in the South Pennine Orefield, central England. J Geol Soc Lond 152:751–765
Ford TD (1967) The stratiform ore deposits of Derbyshire. Proceed 15th Int-University Geol Congress 1967, Univ Leicester, England, pp 73–93
Ford TD (1976) The ores of the South Pennines and Mendip Hills, England—a comparative study. In: Wolf KH (ed) Handbook of stratabound and stratiform ore deposits, 5. Elsevier, Amsterdam, pp 161–196
Fritz P, Frape SK (eds) (1987) Saline water and gases in crystalline rocks. Geol Assoc Can Spec Pap 33, 259 pp
Galindo C, Tornos F, Darbyshire DPF, Casquet C (1994) The age and origin of the barite fluorite (Pb-Zn) veins of the Sierra-Del-Guadarrama (Spanish-Central-System, Spain)—a radiogenic (Nd, Sr) and stable isotope study. Chem Geol 112:351–364
Garven G, Freeze RA (1984) Theoretical analysis of the role of groundwater flow in the genesis of stratabound ore deposits, 1. Mathematical and numerical model. 2. Quantitative results. Am J Sci 284:1085–1174
Gerstenberger H, Haase G (1997) A highly effective emitter substance for mass spectrometric Pb isotope ratio determinations. Chem Geol 136:309–312
Halliday AN (1984) Coupled Sm-Nd and U-Pb systematics in late Caledonian granites and the basement under northern Britain. Nature 307:229–233
Halliday AN, Shepherd TJ, Dickin AP, Chesley JT (1990) Sm-Nd evidence for the age and origin of a Mississippi Valley-type ore deposit. Nature 344:54–56
Hanor JS (1979) The sedimentary genesis of hydrothermal fluids. In: Barnes HL (ed) Geochemistry of hydrothermal ore deposits. Wiley, New York, pp 137–172
Hanor JS (1995) Origin of saline fluids in sedimentary basins. In: Parnell J (ed) Geofluids: origin, migration, and evolution of fluids in sedimentary basins. Geol Soc Spec Publ 78:151–174
Ineson PR (1979) Sulphur, oxygen and carbon isotope investigations of lead-zinc-barite-fluorite-calcite mineralization, Derbyshire, England. Trans Inst Min Metall B88:107–117
Ineson PR, Mitchel JG (1972) Isotopic age determination on clay minerals from lavas and tuffs on the Derbyshire orefield. Geol Mag 109:501–512
Ixer RA, Townley R (1979) The sulphide mineralogy and paragenesis of the South Pennine Orefield, England. Merch Geol 7:51–63
Jaffey AH, Flynn KF, Glendenin LE, Bentley WC, Essling AM (1971) Precision measurement of half-lives and specific activities of 235U and 238U. Phys Rev C4:1889–1906
Jones DG, Plant JA, Colman TB (1991) New evidence for Viséan-Namurian shales as the source of the Pennine mineralisation of England. In: Pagel M, Leroy JL (eds) Source, transport and deposition of metals. Balkema, Rotterdam, pp 309–312
Jones DG, Swainbank IG (1993) Leachate lead isotope studies of potential sources of the South Pennine Orefield of England. In: Fenoll Hach-A, Torres-Ruiz J, Gervilla F (eds) Current research in geology applied to ore deposits. Proceedings 2nd Biennial SGA Meeting, Granada, pp 139–142
Kendrick MA, Burgess R, Pattrick RAD, Turner G (2002) Hydrothermal fluid origins in a fluorite-rich Mississippi Valley-type district: combined noble gas (He, Ar, Kr) and halogen (Cl, Br, I) analysis of fluid inclusions from the South Pennine Ore Field, United Kingdom. Econ Geol 97:435–451
Kesler SE, Appold MS, Martini AM, Walter LM, Huston TJ, Kyle JT (1995) Na-Cl-Br systematics of mineralizing brines in Mississippi Valley-type deposits. Geology 23:641–644
Leach DL, Bradley D, Lewchuk MT, Symons DTA, De Marsily G, Brannon J (2001) Mississippi Valley-type lead-zinc deposits through geological time: implications from recent age-dating research. Miner Deposita 36:711–740
Lüders V, Möller P, Dulski P (1993) REE fractionation in carbonates and fluorite. In: Möller P, Lüders V (eds) Hydrothermal vein deposits. Monogr Ser Mineral Dep 30:133–150
McLennan SM (1989) Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. In: Lipin BR, McKays GA (eds) Rare earth elements. Rev Mineral 21:169–200
Manhès G, Minster JF, Allègre CJ (1978) Comparative uranium-thorium-lead and rubidium-strontium study of the Saint Séverin amphoterite: consequences for early solar system chronology. Earth Planet Sci Lett 39:14–24
Masheder R, Rankin AH (1988) Fluid inclusion studies on the Econ Hill copper deposits, North Staffordshire. Mineral Mag 52:473–482
Möller P (1991) REE fractionation in hydrothermal fluorite and calcite. In: Pagel M, Leroy J (eds) Source, transport and deposition of metals, Balkema, Rotterdam, pp 91–94
Möller P, Bau M (1993) Rare-earth element patterns with positive cerium anomaly in alkaline waters from Lake Van, Turkey. Earth Planet Sci Lett 117:671–676
Möller P, Morteani G (1983) On the geochemical fractionation of rare earth elements during the formation of Ca minerals and its application to problems of the genesis of ore deposits. In: Augustithis SS (ed) The significance of trace elements in solving petrogenetic problems and controversies. Theophrastus Publ, Athens, pp 747–791
Möller P, Bau M, Dulski P, Lüders V (1998) REE and yttrium fractionation in fluorite and their bearing on fluorite formation. Proc 9th Quadr IAGOD Symp, pp 575–592
Moser MR, Rankin AH, Milledge (1992) Hydrocarbon-bearing fluid inclusions in fluorite associated with the Windy Knoll bitumen deposit, UK. Geochim Cosmochim Acta 56:155–168
Mostaghel MA, Ford TD (1986) A sedimentary basin evolution model for ore genesis in the South Pennine Orefield. Mercian Geol10:209–224
Nägler TF, Pettke T, Marshall D (1995) Initial isotopic heterogeneity and secondary disturbance of the Sm-Nd system in fluorites and fluid inclusions: A study on mesothermal veins from the central and western Swiss Alps. Chem Geol 125:241–248
Parekh PP, Möller P, Dulski P (1977) Disribution of trace elements between carbonate and non-carbonate phases of limestone. Earth Planet Sci Lett 34:39–50
Plumlee GS, Goldhaber MS, Rowan EL (1995) The potential role of magmatic gases in the genesis of Illinois-Kentucky fluorspar deposits: Implications from chemical reaction path modeling. Econ Geol 90:999–1011
Robinson BW, Ineson PR (1979) Sulphur, oxygen and carbon isotope investigations of lead-zinc-barite-fluorite-calcite mineralization, Derbyshire, England. Trans Inst Min Metall B88:107–117
Roedder E (1967) Environment of deposition of stratiform (Mississippi Valley-type) ore deposits from studies of fluid inclusions. Econ Geol Monogr 3:349–362
Rogers PJ (1977) Fluid inclusion studies in fluorite from the Derbyshire orefield. Trans Inst Mineral Metall B86:128–132
Sawkins FA (1966) Ore genesis in the North Pennine Orefield, in the light of fluid inclusion studies. Econ Geol 61:385–401
Schneider H-J, Möller P, Parekh PP (1975) Fluorine contents in carbonate sequences and rare earth element distribution in fluorites of the Pb-Zn deposits in East-Alpine Mid-Triassic. Miner Deposita 12:22–36
Shepherd TJ, Darbyshire DPF, Moore GR (1982) Rare earth element and isotopic geochemistry of the North Pennine ore deposits. Bull Bur Mech Gites Min 11:371–377
Steiger RH, Jäger E (1977). Subcommission of geochronology: convention on the use of decay constants in geo- and cosmochronology. Earth Planet Sci Lett 36:359–362
Sverjensky DA (1986) Genesis of Mississippi Valley-type lead-zinc deposits. Annu Rev Earth Planet Sci 14:177–199
Tilton GR (1973) Isotopic lead ages of chondritic meteorites. Earth Planet Sci Lett 19:321–329
Veizer J, Ala D, Azmy K, Bruckschen P, Buhl D, Bruhn F, Carden-Giles AF, Diener A, Ebneth S, Godderis Y, Jasper T, Korte C, Pawellek F, Podlaha OG, Strauss H (1999) 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem Geol 161:59–88
Viets JG, Hofstra AH, Emsbo P (1996) Solute compositions of fluid inclusions in sphalerite from North American and European Mississippi Valley-type ore deposits: ore fluids derived from evaporated seawater. In: Sangster DF (ed) Carbonate-hosted lead-zinc deposits. SEG Spec Publ 4:465–482
Walters SG, Ineson PR (1980) Mineralisation within the igneous rocks of the South Pennine Orefield. Bull Peak Dist Mines Hist Soc 7:315–325
Webb GE, Kamber BS (2002) Rare earth elements in Holocene reefal microbialites; a new shallow seawater proxy. Geochim Cosmochim Acta 64:1557–1565
Williams-Jones AE, Samson IM, Olivo GR (2000) The genesis of hydrothermal fluorite-REE deposits in the Gallinas Mountains, New Mexico. Econ Geol 95:327–341
Acknowledgements
We are most grateful to Peter Möller for introducing us to the fascination and pitfalls of rare earth element geochemistry and for always providing room for independent thinking. We acknowledge the help of N.J.D. Butcher and D.G. Jones for guiding sampling field trips in the South Pennine Orefield. Special thanks go to the manager of the Blue John Caverns for permitting sampling of Blue John fluorite. We thank P. Meier, B. Richert, C. Schulz, C. Wiesenberg, and B. Zander for assistance in the lab. Constructive comments by U. Haack and B. Lehmann helped improve the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: B. Lehmann
Rights and permissions
About this article
Cite this article
Bau, M., Romer, R.L., Lüders, V. et al. Tracing element sources of hydrothermal mineral deposits: REE and Y distribution and Sr-Nd-Pb isotopes in fluorite from MVT deposits in the Pennine Orefield, England. Miner Deposita 38, 992–1008 (2003). https://doi.org/10.1007/s00126-003-0376-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00126-003-0376-x