Abstract
Key message
The Br locus confers bruchid resistance in mungbean; VrPGIP2 (encoding a polygalacturonase inhibitor) is a strong candidate gene for this resistance. The VrPGIP2 sequence differs between resistant and susceptible lines.
Abstract
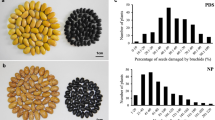
Azuki bean weevil (Callosobruchus chinensis) and cowpea weevil (Callosobruchus maculatus) are serious insect pests of mungbean during storage. Bruchid resistance in mungbean is controlled by a single dominant locus, Br. Although the Br locus has been located on a genetic map, molecular basis and function of the gene remain unknown. In this study, high-resolution mapping using a BC11F2 population of 418 plants derived from a cross between ‘Kamphaeng Saen 1’ (KPS1; susceptible) and ‘V2802’ (resistant) using simple sequence repeat (SSR) markers delimited the Br locus to a genomic region of 38 Kb of chromosome 5 containing two annotated genes. EST-SSR marker DMB-SSR158 co-segregated perfectly with the Br locus. Bioinformatics analyses revealed that DMB-SSR158 corresponds to a gene encoding a polygalacturonase inhibitor (polygalacturonase-inhibiting protein PGIP) and was designated as VrPGIP2. Comparison of VrPGIP2 coding sequences between four bruchid-resistant (V2802, V1128, V2817 and TC1966) and four bruchid-susceptible (KPS1, Sulu-1, CM and an unknown accession) mungbean lines revealed six single nucleotide polymorphisms (SNPs) between the resistant and susceptible groups. Three of the six SNPs resulted in amino acid changes; namely, alanine (A) to serine (S) at position 320, leucine (L) to proline (P) at position 332, and threonine (T) to P at position 335 of the VrPGIP2 sequence in resistant lines, compared with that in susceptible lines. The A to S change at position 320 may affect the interaction between PGIP and polygalacuronase. These results indicate that VrPGIP2 is very likely the gene at the Br locus responsible for bruchid resistance in mungbean.




Similar content being viewed by others
References
Benchimol LL, de Campos T, Carbonell SAM, Colombo CA, Chioratto AF, Formighieri EF, Gouvêa LRL, de Souza AP (2007) Structure of genetic diversity among common bean (Phaseolus vulgaris L.) varieties of Mesoamerican and Andean origins using new developed microsatellite markers. Genet Resour Crop Evol 54:1747–1762
Boyd DW Jr, Cohen AC, Alverson DR (2002) Digestive enzymes and stylet morphology of Deraeocoris nebulosus (Hemiptera: miridae), a predacious plant bug. Ann Entomol Soc Am 95:395–401
Chen HM, Liu CA, Kuo GC, Chien CM, Sun HC, Huang CC, Lin YC, Ku HM (2007) Development of a molecular marker for a bruchid (Callosobruchus chinensis L.) resistance gene in mungbean. Euphytica 157:113–122
Chen HM, Ku HS, Schafleitner R, Bains TS, Kuo GC, Liu CA, Nair RM (2013) The major quantitative trait locus for mungbean yellow mosaic Indian virus resistance is tightly linked in repulsion phase to the major bruchid resistance locus in a cross between mungbean [Vigna radiata (L.) Wilczek] and its wild relative Vigna radiata ssp. sublobata. Euphytica 192:205–216
Chotechung S, Somta P, Chankaew S, Srinives P, Somta P (2011) Identification of DNA markers associated with bruchid resistance in mungbean. Khon Khan Agri J 39(S3):221–226 (in Thai with English abstract)
Development Core Team R (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Frati F, Galletti R, De Lorenzo G, Salerno G, Conti E (2006) Activity of endo- polygalacturonases in mirid bugs (Heteroptera: miridae) and their inhibition by plant cell wall proteins (PGIPs). Eur J Entomol 103:515–522
Fujii K, Miyazaki S (1987) Infestation resistance of wild legumes (Vigna sublobata) to azuki bean weevil, Callosobruchus chinensis (L.) (Coleoptera: bruchidae) and its relationship with cytogenetic classification. Appl Ent Zool 22:229–230
Fujii K, Ishimoto M, Kitamura K (1989) Patterns of resistance to bean weevils (bruchidae) in Vigna radiata-sublobata complex inform the breeding of new resistant varieties. Appl Entomol Zool 24:126–132
Gaitán-Solís E, Duque MC, Edwards KJ, Tohme J (2002) Microsatellite repeats in common bean (Phaseolus vulgaris): isolation, characterization and cross-species amplification in Phaseolus ssp. Crop Sci 42:2128–2136
Girard C, Jouanin L (1999) Molecular cloning of cDNAs encoding a range of digestive enzymes from a phytophagous beetle, Phaedon cochleariae. Insect Biochem Mol Biol 29:1129–1142
Grisi MCM, Blair MW, Gepts P, Brondani C, Pereira PAA, Brondani RPV (2007) Genetic mapping of a new set of microsatellite markers in a reference common bean (Phaseolus vulgaris) population BAT93 × Jalo EEP558. Genet Mol Res 6:691–706
Han OK, Kaga A, Isemura T, Wang XW, Tomooka N, Vaughan DA (2005) A genetic linkage map for azuki bean [Vigna angularis (Willd.) Ohwi & Ohashi]. Theor Appl Genet 111:1278–1287
Hong MG, Kim KH, Ku JH, Jeong JK, Seo MJ, Park CH et al (2015) Inheritance and quantitative trait loci analysis of resistance genes to bruchid and bean bug in mungbean (Vigna radiata L. Wilczek). Plant Breed Biotechnol 3:39–46
Isemura T, Kaga A, Tabata S, Somta P, Srinives P, Shimizu T et al (2012) Construction of a genetic linkage map and genetic analysis of domestication related traits in mungbean (Vigna radiata). PLoS ONE 7:e41304
Ishimoto M, Kitamura K (1989) Growth inhibitory effects of an α-amylase inhibitor from kidney bean, Phaseolus vulgaris (L.) on three species of bruchids (Coleoptera: bruchidae). Appl Ent Zool 24:281–286
Ishimoto M, Sato T, Chrispeels MJ, Kitamura K (2006) Bruchid resistance of transgenic azuki bean expressing seed α-amylase inhibitor of common bean. Entomol Exp Appl 79:309–315
Kaga A, Ishimoto M (1998) Genetic localization of a bruchid resistance gene and its relationship to insecticidal cyclopeptide alkaloids, the vignatic acids in mungbean (V. radiata L.Wilczek). Mol Gen Genet 258:378–384
Kang YJ, Kim S, Kim MY, Lestari P, Kim KH, Ha BK et al (2014) Genome sequence of mungbean and insights into evolution within Vigna species. Nat Commun 5:5443. doi:10.1038/ncomms6443
Kitamura K, Ishimoto M, Sawa M (1988) Inheritance of resistance to infestation with azuki bean weevil in Vigna sublobata and successful incorporation to V. radiata. Jpn J Breed 38:459–464
Kongjaimun A, Kaga A, Tomooka N, Somta P, Shimizu T, Shu Y et al (2012) An SSR-based linkage map of yardlong bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis group) and QTL analysis of pod length. Genome 55:81–92
Lambrides CJ, Imrie BC (2000) Susceptibility of mungbean varieties to the bruchid species Callosobruchus maculatus (F.), C phaseoli (Gyll.), C chinensis (L.), and Acanthoscelides obtectus (Say) (Coleoptera: chrysomelidae). Aust J Agric Res 51:85–89
Lawrence PK, Koundal KR (2002) Plant protease inhibitors in control of phytophagous insects. Electron J Biotechnol 5(1):3. doi:10.2225/vol5-issue1-fulltext-3Electronic
Leckie F, Mattei B, Capodicasa C, Hemmings A, Nuss L, Aracri B et al (1999) The specificity of polygalacturonase-inhibiting protein (PGIP): a single amino acid substitution in the solvent-exposed beta-strand/beta-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J 418:2352–2363
Li H, Ye G, Wang J (2007) A modified algorithm for the improvement of composite interval mapping. Genetics 175:361–374
Liu MS, Kuo TCY, Ko CY, Wu DC, Li KY, Lin WJ et al (2016) Genomic and transcriptomic comparison of nucleotide variations for insights into bruchid resistance of mungbean (Vigna radiata [L.] R. Wilczek). BMC Plant Biol 16:46
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12:6–13
Maréchal R, Mascherpa JM, Stainier F (1978) Etude taxonomiqe d’un group d’espécies des geners Phaseolus et Vigna (Papilionaceae) sur la basedenees morphologiques, traitees, pour 1’ analyse informatique. Boisseira 28:1–278
Mei L, Cheng XZ, Wang SH, Wang LX, Liu CY, Sun L et al (2009) Relationship between bruchid resistance and seed mass in mungbean based on QTL analysis. Genome 52:589–596
Menancio-Hautea D, Kumar L, Danesh D, Young ND (1993) A genome map for mungbean (Vigna radiata L. Wilczek) based on DNA genetic markers (2 N = 2X = 22). In: O’Brien SJ (ed) Genetic maps: locus maps of complex genomes, Cold Spring Harbor Laboratory Press, New York, pp 6.259–6.261
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Miyagi M, Humphry M, Ma ZY, Lambrides CJ, Bateson M, Liu CJ (2004) Construction of bacterial artificial chromosome libraries and their application in developing PCR-based markers closely linked to a major locus conditioning bruchid resistance in mungbean (Vigna radiata L. Wilczek). Theor Appl Genet 110:151–156
Nair RM, Schafleiner R, Kenyon L, Srinivasan R, Easdown W, Ebert AW, Hanson P (2012) Genetic improvement of mungbean. SABRAO J Breed Genet 45:177–190
Nogueira FCS, Silva CP, Alexandre D, Samuels RI, Soares EL, Aragão FJL et al (2012) Global proteome changes in larvae of Callosobruchus maculatus (Coleoptera: chrysomelidae: bruchinae) following ingestion of a cysteine proteinase inhibitor. Proteomics 12:2704–2715
Pauchet Y, Wilkinson P, Chauhan R, Ffrench-Constant RH (2010) Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One 5:e15635
Pedra JHF, Brandt A, Westerman R, Lobo N, Li HM, Romero-Severson J, Murdock LL, Pittendrigh BR (2003) Transcriptome analysis of the cowpea weevil bruchid: identification of putative proteinases and alpha-amylases associated with food breakdown. Insect Mol Biol 12:405–412
Santana MC (2013) Identificação de poligalacturonases expressas no sistema digestório de Callosobruchus maculatus (Coleoptera: chrysomelidae: bruchinae). Ph.D. Thesis, Universidade Federal de Santa Catarina, Brazil
Shade RE, Schroeder HE, Pueyo JJ, Tabe LL, Murdock TJV, Higgins MJ, Chrispeels MJ (1994) Transgenic pea seeds expressing the α-amylase inhibitor of the common bean are resistant to bruchid beetles. Bio/Technol 12:793–796
Shen Z, Reese JC, Reeck GR (1996) Purification and characterization of polygalacturonase from the rice weevil, Sitophilus oryzae (Colecoptera: curculionidae). Insect Biochem Mol Biol 26:427–433
Somta P, Srinives P (2007) Genome research in mungbean [Vigna radiata (L.) Wilczek] and blackgram [V. mungo (L.) Hepper]. Sci Asia 33(S1):69–74
Somta P, Ammaranan C, Ooi PAC, Srinives P (2007) Inheritance of seed resistance to bruchids in cultivated mungbean (Vigna radiata (L) Wilzcek). Euphytica 155:49–55
Somta C, Somta P, Tomooka N, Ooi PAC, Vaughan DA, Srinives P (2008) Characterization of new sources of mungbean (Vigna radiata (L) Wilczek) resistance to bruchids, Callosobruchus spp (Coleoptera: bruchidae). J Stored Prod Res 44:316–321
Somta P, Seehalak W, Srinives P (2009) Development, characterization and cross-species amplifcation of mungbean (Vigna radiata) genic microsatellite markers. Conserv Genet 10:1939–1943
Srinives P, Somta P, Somta C (2007) Genetics and breeding of resistance to bruchids (Callosobruchus spp) in Vigna crops: a review. NU Int J Sci 4:1–17
Sun L, Cheng XZ, Wang SH, Wang LX, Liu CY, Mei L, Xu N (2008) Heredity analysis and gene mapping of bruchid resistance of a mungbean cultivar V2709. Agric Sci China 7:672–677
Talekar NS, Lin CL (1992) Characterization of Callosobruchus chinensis (Coleoptera: bruchidae) resistance in mungbean. J Econ Entomol 85:1150–1153
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11:1441–1452
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3—new capabilities and interfaces. Nucleic Acids Res 40(15):e115
Young N, Kumar L, Menancio-Hautea D, Danesh D, Talekar NS, Shanmugasundaram S, Kim DH (1992) RFLP mapping of a major bruchid resistance gene in mungbean (Vigna radiata, L Wilczek). Theor Appl Genet 84:839–844
Yu K, Park SJ, Poysa V, Gepts P (2000) Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J Hered 91:429–434
Zhao D, Cheng XZ, Wang LX, Wang SH, Ma YL (2010) Integration of mungbean (Vigna radiata) genetic linkage map. Acta Agron Sin 36:932–939
Acknowledgments
This research was funded by the Center for Advanced Studies for Agriculture and Food (CASAF), Kasetsart University under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand to P. Somta. We also acknowledge initial support from Thailand’s National Science and Technology Development Agency to P. Srinives.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
The experiments carried out in this study comply with the current laws of Thailand.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Matthew N Nelson.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chotechung, S., Somta, P., Chen, J. et al. A gene encoding a polygalacturonase-inhibiting protein (PGIP) is a candidate gene for bruchid (Coleoptera: bruchidae) resistance in mungbean (Vigna radiata). Theor Appl Genet 129, 1673–1683 (2016). https://doi.org/10.1007/s00122-016-2731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-016-2731-1