Abstract
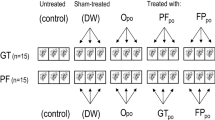
The preen gland is a holocrine sebaceous gland of the avian integument which produces an oily secretion that is spread on the plumage during preening. It has been suggested that birds may defend themselves against feather-degrading bacteria (FDB) and other potential pathogens using preen gland secretions. However, besides some in vitro studies, the in vivo bacterial inhibitory effects of the preen oil on the abundance of feather-associated bacterial species has not yet been studied in passerines. Here we tested the effect of gland removal on the abundance of FDB and other-cultivable bacterial loads (OCB) of male house sparrows (Passer domesticus). Our results did not support earlier results on in vitro antibacterial activity of preen oil against FDB since the absence of the preen gland did not significantly affect their loads related to the control birds. In contrast, we found that preen gland removal led to higher loads of OCB. This result suggests that the antimicrobial spectrum of the preen oil is broader than previously thought and that, by reducing the overall feather bacterial loads, the preen gland could help birds to protect themselves against a variety of potentially harmful bacteria.

Similar content being viewed by others
References
Achá SJ, Kühn I, Mbazima G, Colque-Navarro P, Möllby R (2005) Changes of viability and composition of the Escherichia coli flora in faecal samples during long time storage. J Microbiol Meth 63:229–238
Bandyopadhyay A, Bhattacharyya SP (1996) Influence of fowl uropygial gland and its secretory lipid components on the growth of skin surface bacteria of fowl. Indian J Exp Biol 34:48–52
Bisson I-A, Marra PP, Burtt EH, Sikaroodi M, Gillevet PM (2007) A molecular comparison of plumage and soil bacteria across biogeographic, ecological, and taxonomic scales. Microb Ecol 54:65–81
Bisson I-A, Marra PP, Burtt EH, Sikaroodi M, Gillevet PM (2009) Variation in plumage microbiota depends on season and migration. Microb Ecol 58:212–220
Burger BV, Reiter B, Borzyk O, du Plessis MA (2004) Avian exocrine secretions: I. Chemical characterization of the volatile fraction of the uropygial secretion of the green woodhoopoe, Phoeniculus purpureus. J Chem Ecol 30:1603–1611
Burtt EH Jr, Ichida JM (1999) Occurrence of feather-degrading bacilli in the plumage of birds. Auk 116:364–372
Campagna S, Mardon J, Celerier A, Bonadonna F (2012) Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years studies. Chem Senses 37:3–25
Clayton DH (1999) Feather-busting bacteria. Auk 116:302–304
Clayton DH, Koop JAH, Harbison CW, Moyer BR, Bush SE (2010) How birds combat ectoparasites. Open Ornithol J 3:41–71
Cook MI, Beissinger SR, Toranzos GA, Arendt WJ (2005) Incubation reduces microbial growth on eggshells and the opportunity for trans-shell infection. Ecol Lett 8:532–537
Cristol DA, Armstrong JL, Whitaker JM, Forsyth MH (2005) Feather-degrading bacteria do not affect feathers on captive birds. Auk 122:222–230
Czirják GÁ, Møller AP, Mousseau TA, Heeb P (2010) Microorganisms associated with feathers of barn swallows in radioactively contaminated areas around Chernobyl. Microb Ecol 60:373–380
Galván I, Barba E, Piculo R, Cantó JL, Cortés V, Monrós JS, Atiénzar F, Proctor H (2008) Feather mites and birds: an interaction mediated by uropygial gland size? J Evol Biol 21:133–144
Giraudeau M, Duval C, Guillon N, Bretagnolle V, Gutierrez C, Heeb P (2010a) Effects of access to preen gland secretions on mallard plumage. Naturwissenschaften 97:577–581
Giraudeau M, Czirják GÁ, Duval C, Bretagnolle V, Eraud C, McGraw KJ, Heeb P (2010b) Effect of restricted preen-gland access on maternal self maintenance and reproductive investment in mallards. PLoS One 5:e13555
Giraudeau M, Czirják GÁ, Duval C, Bretagnolle V, Gutierrez C, Guillon N, Heeb P (2013) Effect of preen oil on plumage bacteria: an experimental test with the mallard. Behav Process in press
Gunderson AR (2008) Feather-degrading bacteria: a new frontier in avian and host–parasite research? Auk 125:972–979
Gunderson AR, Forsyth MH, Swaddle JP (2009) Evidence that plumage bacteria influence feather coloration and body condition of eastern bluebirds Sialia sialis. J Avian Biol 40:440–447
Hubálek Z (2004) An annotated checklist of pathogenic microorganisms associated with migratory birds. J Wildl Dis 40:639–659
Jacob J, Ziswiler V (1982) The uropygial gland. In: Farner DS, King JR, Parkes KC (eds) Avian biology, vol 6. Academic Press, New York, pp 199–324
Jacob J, Eigener U, Hoppe U (1997) The structure of preen gland waxes from pelecaniform birds containing 3,7-dimethyloctan-1-ol: an active ingredient against dermatophytes. Z Naturforsch 52:114–123
Leclaire S, Merkling T, Raynaud C, Giacinti G, Bessière J-M, Hatch SA, Danchin É (2011) An individual and a sex odor signature in kittiwakes? Study of the semiochemical composition of preen secretion and preen down feathers. Naturwissenschaften 98:615–624
López-Rull I, Pagán I, Garcia CM (2010) Cosmetic enhancement of signal coloration: experimental evidence in the house finch. Behav Ecol 21:781–787
Lucas FS, Heeb P (2005) Environmental factors shape cloacal bacteria assemblages in great tit Parus major and blue tit P. caeruleus nestlings. J Avian Biol 36:510–516
Lucas FS, Broennimann O, Febbraro I, Heeb P (2003) High diversity among feather-degrading bacteria from a dry meadow soil. Microb Ecol 45:282–290
Mallinson ET, Tate CR, Miller RG, Bennett B, Russek-Cohen E (1989) Monitoring poultry farms for Salmonella by drag-swab sampling and antigen-capture immunoassay. Avian Dis 33:684–690
Martín-Vivaldi M, Ruiz-Rodríguez M, Soler JJ, Peralta-Sánchez JM, Méndez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M (2009) Seasonal, sexual and developmental differences in hoopoe Upupa epops preen gland morphology and secretions: evidence for a role of bacteria. J Avian Biol 40:191–205
Mennerat A, Mirleau P, Blondel J, Perret P, Lambrechts MM, Heeb P (2009) Aromatic plants in nests of the blue tit Cyanistes caeruleus protect chicks from bacteria. Oecologia 161:849–855
Møller AP, Czirják GÁ, Heeb P (2009) Feather micro-organisms and uropygial antimicrobial defences in a colonial passerine bird. Funct Ecol 23:1097–1102
Møller AP, Erritzøe J, Nielsen JT (2010) Predators and microorganisms of prey: goshawks prefer prey with small uropygial glands. Funct Ecol 24:608–613
Montalti D, Gutiérrez AM, Reboredo GR, Salibián A (2006) Removal of the uropygial gland does not affect serum lipids, cholesterol and calcium levels in the rock pigeon Columba livia. Acta Biol Hung 57:295–300
Moreno J, Briones V, Merino S, Ballesteros C, Sanz JJ, Tomás G (2003) Beneficial effects of cloacal bacteria on growth and fledging size in nestling Pied Flycatchers (Ficedula hypoleuca) in Spain. Auk 120:784–790
Moreno-Rueda G (2010) Uropygial gland size correlates with feather holes, body condition and wingbar size in the house sparrow Passer domesticus. J Avian Biol 41:229–236
Moreno-Rueda G (2011) House sparrows Passer domesticus with larger uropygial glands show reduced feather wear. Ibis 153:195–198
Moyer BR, Rock AN, Clayton DH (2003) Experimental test of the importance of preen oil in Rock Doves (Columba livia). Auk 120:490–496
Mureşan C, Pap PL, Czirják GÁ, Bolfa P (2009) Excision of the uropygial gland in the house sparrow. Lucr Şt Med Vet Timişoara XLII:111–114
Muza MM, Burtt EH Jr, Ichida JM (2000) Distribution of bacteria on feathers of some Eastern North American birds. Wilson Bull 112:432–435
Pap PL, Vágási CI, Osváth G, Mureşan C, Barta Z (2010a) Seasonality in the uropygial gland size and feather mite abundance in house sparrows: natural covariation and an experiment. J Avian Biol 41:653–661
Pap PL, Czirják GÁ, Vágási CI, Barta Z, Hasselquist D (2010b) Sexual dimorphism in immune function changes during the annual cycle in the house sparrows. Naturwissenschaften 97:891–901
Peralta-Sánchez JM, Møller AP, Martín-Platero AM, Soler JJ (2010) Number and colour composition of nest lining feathers predict eggshell bacterial community in barn swallow nests: an experimental study. Funct Ecol 24:426–433
Piersma T, Dekker M, Sinninghe Damsté JS (1999) An avian equivalent of make-up? Ecol Lett 2:201–203
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, http://www.R-project.org
Reneerkens J, Piersma T, Sinninghe Damsté JS (2005) Switch to diester preen waxes may reduce avian nest predation by mammalian predators using olfactory cues. J Exp Biol 208:4199–4202
Reneerkens J, Versteegh MA, Schneider AM, Piersma T, Burtt EH Jr (2008) Seasonally changing preen-wax composition: Red Knots’ (Calidris canutus) flexible defense against feather-degrading bacteria? Auk 125:285–290
Ruiz-De-Castañeda R, Vela AI, González-Braojos S, Briones V, Moreno J (2011) Drying eggs to inhibit bacteria: incubation during laying in a cavity nesting passerine. Behav Process 88:142–148
Ruiz-Rodríguez M, Lucas FS, Heeb P, Soler JJ (2009a) Differences in intestinal microbiota between avian brood parasites and their hosts. Biol J Linn Soc 96:406–414
Ruiz-Rodríguez M, Soler JJ, Lucas FS, Heeb P, Palacios MS, Martín-Gálvez D, de Neve L, Pérez-Contreras T, Martínez JG, Soler M (2009b) Bacterial diversity at the cloaca relates to an immune response in magpie Pica pica and to body condition of great spotted cuckoo Clamator glandarius nestlings. J Avian Biol 40:42–48
Saranathan V, Burtt EH Jr (2007) Sunlight on feathers inhibits feather-degrading bacteria. Wilson J Ornithol 119:239–245
Shawkey MD, Mills KL, Dale C, Hill GE (2005) Microbial diversity of wild bird feathers revealed through culture-based and culture-independent techniques. Microb Ecol 50:40–47
Shawkey MD, Pillai SR, Hill GE (2003) Chemical warfare? Effects of uropygial oil on feather-degrading bacteria. J Avian Biol 34:345–349
Shawkey MD, Pillai SR, Hill GE, Siefferman LM, Roberts SR (2007) Bacteria as an agent for change in structural plumage color: correlational and experimental evidence. Am Nat 169:S112–S121
Shawkey MD, Firestone MK, Brodie EL, Beissinger SR (2009a) Avian incubation inhibits growth and diversification of bacterial assemblages on eggs. PLoS One 4:e4522
Shawkey MD, Pillai SR, Hill GE (2009b) Do feather-degrading bacteria affect sexually selected plumage color? Naturwissenschaften 96:123–128
Soini HA, Schrock SE, Bruce KE, Wiesler D, Ketterson ED, Novotny MV (2007) Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J Chem Ecol 33:183–198
Soler JJ, Martín-Vivaldi M, Ruiz-Rodríguez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M, Peralta-Sánchez JM, Méndez M (2008) Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct Ecol 22:864–871
Soler JJ, Peralta-Sánchez JM, Flensted-Jensen E, Martín-Platero AM, Møller AP (2011) Innate humoural immunity is related to eggshell bacterial load of European birds: a comparative analysis. Naturwissenschaften 98:807–813
Soler JJ, Peralta-Sánchez JM, Martín-Platero AM, Martín-Vivaldi M, Martínez-Bueno M, Møller AP (2012) The evolution of size of the uropygial gland: mutualistic feather mites and uropygial secretion reduce bacterial loads of eggshells and hatching failures of European birds. J Evol Biol 25:1179–1191
Whitaker JM, Cristol DA, Forsyth MH (2005) Prevalence and genetic diversity of Bacillus licheniformis in avian plumage. J Field Ornithol 76:264–270
Whittaker DJ, Richmond KM, Miller AK, Kiley R, Bergeon Burns C, Atwell JW, Ketterson ED (2011) Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav Ecol 22:1256–1263
White J, Mirleau P, Danchin E, Mulard H, Hatch SA, Heeb P, Wagner RH (2010) Sexually transmitted bacteria affect female cloacal assemblages in a wild bird. Ecol Lett 13:1515–1524
Acknowledgments
We thank Saria Industries Bretagne S.A.S. France for kindly providing the hydrolyzed feather meal used in this study, and Gábor and Klára Czirják for providing food for the birds. Richard K. Simpson kindly corrected our English. This project was financially supported by a French research grant from the ANR to PH and Marcel M. Lambrechts (ANR-05, NT05-3_42075), an Eiffel grant from the French Ministry of Foreign Affairs to GÁC, a research grant (CEEX ET #94/2006) of the Romanian Ministry of Education and Research to PLP, a PhD scholarship from the Hungarian Ministry of Education and Culture to CIV, and a PhD scholarship from the French Government to MG. The research was supported by the “Laboratoire d’Excellence” TULIP (ANR-10-LABX-41). We thank three anonymous reviewers who provided constructive criticism to an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Alexandre Roulin
Rights and permissions
About this article
Cite this article
Czirják, G.Á., Pap, P.L., Vágási, C.I. et al. Preen gland removal increases plumage bacterial load but not that of feather-degrading bacteria. Naturwissenschaften 100, 145–151 (2013). https://doi.org/10.1007/s00114-012-1005-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-012-1005-2