Abstract
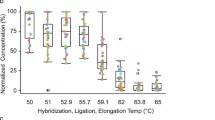
We describe a polymerase chain reaction (PCR) that allowed detection of rRNA consensus sequences from the DNA extracted from a wide range of bacterial species in amounts as low as 10 fg. To avoid false-positive results with universal primers for 16S rRNA PCR, contaminating DNA had to be eliminated from the polymerase preparations. Decontamination was undertaken before PCR to optimize treatment with DNase I and was followed by DNase inactivation at 94°C for 50 min, which eliminated contaminating DNA at concentrations of up to 100 pg. After optimization of PCR conditions for each polymerase, Deep-Vent Exo-®polymerase (New England Biolabs, Beverly, MA), and super-Taq® polymerase (HT Biotechnology, Cambridge, UK) were more effective than Ampli-Taq® polymerase (Perkin-Elmer Cetus, Norwalk, CT), Ampli-Taq LD® polymerase (Perkin-Elmer Cetus) or Deep-vent® polymerase (New England Biolabs). The technique described in this article might prove to be a universal method for PCR detection of small numbers of unidentified bacteria in usually sterile clinical sites, such as blood and cerebrospinal fluids, in which a broad spectrum of pathogens can be expected.
Similar content being viewed by others
References
Medlin, L. H., Elwood, J., Stickel, S., and Sogin, M. L. (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions.Gene 71, 491–499.
Woese, C. R. (1987) Bacterial evolution.Microbiol. Rev. 51, 221–271.
Lane, D. J., Pace, B., Olsen, G. J., Stahl, D. A., Sogin, M. L., and Pace, N. R. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses.Proc. Natl. Acad. Sci. USA 82, 6955–6959.
Böttger, E. C. (1989) Rapid determination of bacterial ribosomal RNA sequences by direct sequencing of enzymatically amplified DNA.FEMS Microbiol. Lett. 65, 171–176.
Weisburg, W. G., Barns, S. M., Pelletier, D. A., and Lane, D. J. (1991) 16S ribosomal DNA amplification for phylogenetic study.J. Bacteriol. 173, 697–703.
Wilson, K. H., Blitchington, R., and Greene, R. C. (1990) Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction.J. Clin. Microbiol. 28, 1942–1946.
Relman, D. A., Loutit, J. S., Schmidt, T. M., Falkow, S., and Tompkins, L. S. (1990) The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens.N. Engl. J. Med. 323, 1573–1583.
Relman, D. A., Schmidt, T. M., MacDermott, R. P., and Falkow, S. (1992) Identification of uncultured Bacillus of Whipple's Disease.N. Engl. J. Med. 323, 293–301.
Kwok, S. and Higuchi, R. (1989). Avoiding false positives with PCR.Nature (Lond.) 339, 237, 238.
Böttger, E. C. (1990) Frequent contamination of Taq polymerase with DNA.Clin. Chem. 36, 1258, 1259.
Schmidt, T. M., Pace, B., and Pace, N. R. (1991) Detection of DNA contamination in Taq polymerase.BioTechniques 11, 176,177.
Meier, A., Persing, D. H., Finken, M., and Böttger, E. C. (1993) Elimination of contaminating DNA within polymerase chain reaction reagents: implications for a general approach to detection of uncultured pathogens.J. Clin. Microbiol. 31, 646–652.
Rys, P. N. and Persing, D. H. (1993) Preventing false positives: quantitative evaluation of three protocols for inactivation of polymerase chain reaction amplification products.J. Clin. Microbiol. 31, 2356–2360.
Widjojoatmodjo, M. N., Fluit, A. D. C., and Verhoef, J. (1994) Rapid identification of bacteria by PCR-single-strand conformation polymorphism.J. Clin. Microbiol. 32, 3002–3007.
Widjojoatmodjo, M. N., Fluit, A. D. C., and Verhoef, J. (1995) Molecular identification of bacteria by fluorescent-based PCR-single-strand conformation polymorphism analysis of the 16S rRNA gene.J. Clin. Microbiol. 33, 2601–2606.
Sahn, D. F., Kissinger, J., Gilmore, M. S., Murray, P. R., Mulder, R., Solliday, J., and Clarke, B. (1989) In vitro susceptibility studies of vancomycin-resistantEnterococcus faecalis.Antimicrob. Agents Chemother. 33, 1588–1591.
Dutka-Malen, S., Leclercq, R., Coutant, V., Duval, J., and Courvalin, P. (1990) Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in Gram-positive bacteria.Antimicrob. Agents Chemother. 34, 1875–1879.
Raleigh, E. A., Murray, N. E., Revel, H., Blumenthal, R. M., Westaway, D., Reith, A. D., Rigby, P. W. J., Ethai, J., and Hanahan, D. (1987) Mcr A and Mcr B restriction phenotypes of someE. coli strains and implications for gene cloning.Nucleic Acids Res. 16, 1563–1575.
Böddinghaus, B., Rogall, T., Flohr, T., Blöcker, H., and Böttger, E. C. (1990) Detection and identification of mycobacteria by amplification of rRNA.J. Clin. Microbiol. 28, 1751–1759.
Boom, R., Sol, C. J. A., Salimans, M. M. M., Jansen, C. L., Wertheim-Van Dillen, P. M. E., and Van der Noordaa, J. (1990) Rapid and simple method for purification of nucleic acids.J. Clin. Microbiol. 28, 495–503.
Manchester, K. L. (1996) Use of UV methods for measurement of protein and nucleic acid concentrations.Biotechniques 20, 968–970.
Edwards, U., Rogall, T., Blöcker, H., Emde, M., and Böttger, E. C. (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA.Nucleic Acids Res. 17, 7843–7853.
Brosius, J., Palmer, M. L., Kennedy, P. J., and Noller, H. F. (1978) Complete nucleotide sequence of a 16S Ribosomal RNA gene fromEscherichia coli.Proc. Natl. Acad. Sci. USA 75, 4801–4805.
Erlich, H. A. (ed.) (1989)PCR Technology, Principles and Applications for DNA amplification. Stockton, England.
Chevet, E., Lemaître, G., and Katinka, M. D. (1995) Low concentrations of tetramethylammonium chloride increase yield and specificity of PCR.Nucleic Acids Res. 23, 3343, 3344.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hilali, F., Saulnier, P., Chachaty, E. et al. Decontamination of polymerase chain reaction reagents for detection of low concentrations of 16S rRNA genes. Mol Biotechnol 7, 207–216 (1997). https://doi.org/10.1007/BF02740812
Issue Date:
DOI: https://doi.org/10.1007/BF02740812