Summary
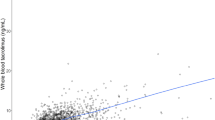
We have measured total and unbound plasma concentrations of cyclosporin A in seven healthy men after single oral doses (12 mg per kg body weight) on two occasions at least two weeks apart.
There was an up to two-fold intraindividual and a more than three-fold interindividual variation in the AUCs of both total and unbound drug.
The intraindividual variability in the AUC of cyclosporin is similar to that of many other drugs and needs to be taken into account in the planning of pharmacokinetic studies.
Similar content being viewed by others
References
Calne RY, White DJG, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Pentlow BD, Rolles K (1978) Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 2: 1323–1327
The Canadian Multicentre Transplant Study Group (1983) A randomized clinical trial of cyclosporine in cadaveric renal transplantation. N Engl J Med 309: 809–815
European Multicentre Trial Group (1982) Cyclosporin A as sole immunosuppressive agent in recipients of kidney allografts from cadaver donors: Preliminary results of a European multicentre trial. Lancet 2: 57–60
Cohen DJ, Loertscher R, Rubin MF, Tilney NL, Carpenter CB, Strom TB (1984) Cyclosporine: A new immunosuppressive agent for organ transplantation. Ann Int Med 101: 667–682
Beveridge T, Gratwohl A, Michot F, Niederberger W, Nüesch E, Nussbaumer K, Schaub P, Speck B (1981) Cyclosporin A: Pharmacokinetics after a single dose in man and serum levels after multiple dosing in recipients of allogeneic bone-marrow grafts. Curr Ther Res 30: 5–18
Robson S, Neuberger J, Keller HP, Abisch E, Niederberger W, von Graffenried B, Williams R (1984) Pharmacokinetic study of cyclosporin A in patients with primary biliary cirrhosis. Br J Clin Pharmacol 18: 627–631
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR (1985) Cyclosporine kinetics in renal transplantation. Clin Pharmacol Ther 38: 296–300
Ptachcinski RJ, Venkataramanan R, Rosenthal JT, Burckart GJ, Taylor RJ, Hakala TR (1985) The effect of food on cyclosporine absorption. Transplantation 40: 174–176
Bertault-Pérès P, Maraninchi D, Carcassonne Y, Cano JP, Barbet J (1985) Clinical pharmacokinetics of cyclosporin A in bone marrow transplant patients. Cancer Chemother Pharmacol 15: 76–81
Burckart GJ, Venkataramanan R, Ptachcinski RJ, Starzl TE, Gartner JC, Zitelli BJ, Malatack JJ, Shaw BW, Iwatsuki S, van Thiel DH (1986) Cyclosporine absorption following orthotopic liver transplantation. J Clin Pharmacol 26: 647–651
Kahan BD, Kramer WG, Wideman C, Flechner SM, Lorber ML, van Buren CT (1986) Demographic factors affecting the pharmacokinetics of cyclosporine estimated by radioimmunoassay. Transplantation 41: 459–464
Tufveson G, Odlind B, Sjöberg O, Lindberg A, Gabrielsson J, Lindström B, Lithell H, Selinus I, Töttermann T, Wahlberg J (1986) A longitudinal study of the pharmacokinetics of cyclosporine A and in vitro lymphocyte responses in renal transplantation. Transplant Proc 18: 16–24
Grevel J, Nüesch E, Abisch E, Kutz K (1986) Pharmacokinetics of oral cyclosporin A (Sandimmun) in healthy subjects. Eur J Clin Pharmacol 31: 211–216
Henricsson S (1987) A new method for measuring the free fraction of cyclosporin in plasma by equilibrium dialysis. J Pharm Pharmacol 39: 384–385
Hows JM, Smith JM (1983) In vitro stability of cyclosporin? J Clin Pathol 36: 720–721
Hamilton G, Roth E, Wallisch E, Tichy F (1985) Semi-automated high-performance liquid chromatographic determination of cyclosporine A in whole blood using one-step sample purification and column-switching. J Chromatogr 341: 411–419
Ericzon BG, Todo S, Lynch I, Kam I, Pfachcinski RJ, Burkkart GJ, van Thiel DH, Starzl TE, Venkataramanan R (1987) Role of bile salts on cyclosporine absorption in dogs. Transplant Proc 19 no. 1: 1248–1249
Andrews W, Iwatsuki S, Starzl TE (1985) Correspondence. Transplantation 39: 338
Venkataramanan R, Burckart GJ, Ptachcinski RJ (1985) Pharmacokinetics and monitoring of cyclosporine following orthotopic liver transplantation. Semin Liver Dis 5: 357–368
Burckart GJ, Starzl TE, Venkataramanan R, Hashim H, Wong L, Wang P, Makowka L, Zeevi A, Ptachcinski RJ, Knapp JE, Iwatsuki S, Esquivel C, Sanghvi A, van Thiel DH (1986) Excretion of cyclosporine and its metabolites in human bile. Transplant Proc 18, no.6 [Suppl 5]: 46–49
Grahnén A (1985) The impact of time-dependent phenomena on bioequivalence studies. In: Breimer DD, Speiser P (eds) Topics in pharmaceutical sciences. Elsevier, Amsterdam, pp 179–190
Gammans RE, Mackenthun AV, Russell JW (1984) Comparative bioavailability of trazodone formulations using stable isotope methodology. Br J Clin Pharmacol 18: 431–437
Grahnén A, Hammarlund M, Lundqvist T (1984) Implications of intraindividual variability in bioavailability studies of furosemide. Eur J Clin Pharmacol 27: 595–602
Schrogie JJ, Davies RO, Hwang SS, Hesney M, Breault GO, Kwan KC, Huber PB, Feinberg JA, Abrams WB, Zinny MA (1979) Intrasubject variability in methyldopa bioavailability. Clin Pharmacol Ther 25: 248
Alván G, Siwers B, Vessman J (1977) Pharmacokinetics of oxazepam in healthy volunteers. Acta Pharmacol Toxicol 40: 40–51
Alvares AP, Kappas A, Eiseman JL, Anderson KE, Pantuck CB, Pantuck EJ, Hsiao K-C, Garland WA, Conney AH (1979) Intraindividual variation in drug disposition. Clin Pharmacol Ther 26: 407–419
Reed CR, Schwartz HJ (1984) Lack of influence of an intensive antacid regimen on theophylline bioavailability. J Pharmacokinet Biopharm 12: 315–331
Lemaire M, Tillement JP (1982) Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J Pharm Pharmacol 34: 715–718
Lithell H, Odlind B, Selinus I, Lindberg A, Lindström B, Frödin L (1986) Is plasma lipoprotein pattern of importance for treatment with cyclosporine? Transplant Proc 18, no. 1: 50–51
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lindholm, A., Henricsson, S., Lind, M. et al. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing. Eur J Clin Pharmacol 34, 461–464 (1988). https://doi.org/10.1007/BF01046702
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01046702