Abstract
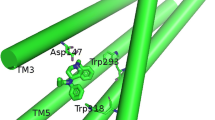
In this model-building study a model for the pore of the acetylcholine receptor channel is proposed. The pore is formed by five α-helices of the M2 segment where three rings of hydrophilic side chains point into the channel lumen. This model is in agreement with most experimental data like photolabeling, drug affinity studies, single channel conductivity measurements and cryo electron microscopy known about this channel.
This study predicts a strong coupling of the motion of the ions in the channel to that of the charged and highly hydrophilic amino acid side chains at the channel wall. Due to the negative net-charge in the pore more than a single cation may occupy the pore region. The resulting strong local electric fields make the commonly used constant field approximation obsolete for this type of ion channel.
Similar content being viewed by others
References
F. Franciolini and A. Petris:Mol. Biol. Evol. 6, 503–513 (1989).
V. B. Cockcroft, D. J. Osguthorpe, E. A. Barnard, and G. G. Lunt:Proteins 8, 129–169 (1990).
V. A. Parsegian:Nature 221, 844–846 (1969).
V. A. Parsegian:Ann. New York Acad. Sci. 264, 161–174 (1975).
R. S. Eisenberg:J. Membrane Biol. 115, 1–12 (1990).
O. S. Andersen and R. E. Koeppe II:Physiol. Rev. 72, S89-S158 (1992).
B. Hille:Ionic Channels of Exitable Membranes, Sinauer Associates Inc., Sunderland, Massachusetts (1992).
E. A. Barnard:Trends Biochem. Sci. 17, 368–374 (1992).
W. A. Catterall:Science 242, 50–60 (1988).
A. V. Maricq, A. S. Peterson, A. J. Brake, R. M. Myers, and D. Julius:Science 254, 432 (1991).
V. B. Cockcroft, D. J. Osguthorpe, E. A. Barnard, A. E. Friday, and G. G. Lunt:Mol. Neurobiol. 4, 129–169 (1990).
J. Deisenhofer, O. Epp, K. Miki, R. Huber, and H. Michel:J. Mol. Biol. 180, 385–398 (1984).
H. Michel and J. Deisenhofer:Chem. Scripta 27B, 173–180 (1987).
C. Toyoshima and N. Unwin:Nature 336, 247–250 (1988).
N. Unwin:Neuron 3, 665–676 (1989).
A. K. Mitra, M. P. McCarthy and R. M. Stroud:J. Cell Biol. 109, 755–774 (1990).
N. Unwin:J. Mol. Biol. 229, 1101–1124 (1993).
H. Betz:Biochem. 29, 3591–3599 (1990).
J. Finer-Moore and R. M. Stroud:Proc. Natl. Acad. Sci. USA 81, 155–159 (1984).
G. Spach, H. Duclohier, G. Molle and J.-M. Valleton:Biochemie 71, 11–21 (1989).
J.-P. Changeux, J.-L. Galzi, A. Devillers-Thiry, and D. Bertrand:Quart. Rev. Biophys. 24, 395–432 (1992)
C. Miller:Neuron 2, 1195–1205 (1989).
J.-L. Galzi, A. Devillers-Thiry, N. Hussy, S. Bertrand, J.-P. Changeux, and D. Bertrand:Nature 359, 500–505 (1992).
A. Karlin:The Harvey Lecture Series 85, 71–107 (1991).
T. Konno, C. Busch, E. von Kitzing, K. Imoto, F. Wang, J. Nakai, M. Mishina, S. Numa, and B. Sakmann:Proc. R. Soc. Lond. B 244, 69–79 (1991).
M. Noda, H. Takahashi, T. Tanabe, M. Toyosato, Y. Furutani, T. Hirose, M. Asai, S. Jinahama, T. Miyata, and S. Numa:Nature 299, 793–797 (1982).
T. Claudio, M. Ballivet, J. Patrick, and S. Heineman:Proc. Natl. Acad. Sci. USA 80, 1111–1115 (1983).
A. Devillers-Thiery, J. Giraudat, M. Bentaboulet, and J.-P. Changeux:Proc. Natl. Acad. Sci. USA 80, 2067–2071 (1983).
H. R. Guy and F. Hucho:Trends Neurosci. 10, 318–321 (1987).
K. Imoto, C. Methfessel, B. Sackmann, M. Mishina, Y. Mori, T. Konno, K. Fukuda, M. Kurasaki, H. Bujo, Y. Fujita, and S. Numa:Nature 324, 670–674 (1986).
R.J. Leonard, C. G. Labarca, P. Chanet, N. Davidson, and H. A. Lester:Science 242, 1578–1581 (1988).
P. Charnet, C. Labarca, R. J. Leonard, N. Vogelaar, L. Czyzyk, A. Gouin, N. Davidson, and H. Lester:Neuron 2, 87–95 (1990).
V. Witzemann, E. Stein, B. Barg, T. Konno, M. Koenen, W. Kues, M. Criado, M. Hofmann, and E. Sakmann:Eur. J. Biochem. 194, 437–448 (1990).
K. Imoto, C. Busch, B. Sackmann, M. Mishina, T. Konno, J. Nakai, H. Bujo, Y. Mori, K. Fukuda, and S. Numa:Nature 335, 645–648 (1988).
F. Wang and K. Imoto:Proc. R. Soc. Lond. B 250, 11–17 (1992).
A. Villarroel, S. Herlitze, M. Koenen, and B. Sakmann:Proc. R. Soc. Lond. B 243, 69–74 (1991).
K. Imoto, T. Konno, J. Nakai, F. Wang, M. Mishina, and S. Numa:FEBS Letters 289, 193–200 (1991).
A. Villarroel and B. Sakmann:Biophys. J. 62, 196–208 (1992).
H. A. Lester:Ann. Rev. Biophys. Biomol. Struct. 21, 267–292 (1992).
A. Devillers-Thiery, J.-L. Galzi, J. L. Eisele, S. Bertrand, D. Bertrand, and J.-P. Changeux:J. Membrane Biol. 136, 97–112 (1993).
F. Hucho and R. Hilgenfeld:FEBS Letters 257, 17–23 (1989).
S. Furois-Corbin and A. Pullman:Biochim. Biophys. Acta 984, 339–350 (1989).
R. M. Stroud, M. McCarthy, and M. Shuster:Biochem. 29, 11009–11023 (1990).
G. Eisenman, A. Villarroel, M. Montal, and O. Alvarez: ‘Energy profiles for ion permeation in pentameric protein channels: from viruses to receptor channels’, in J. M. Ritchie, P. J. Magistretti and L. Bolis (eds.),Progress in Cell Research, Springer Verlag, New York, pp. 195–211 (1990).
A. Pullman:Chem. Rev. 91, 793–812 (1991).
E. A. Barnard, M. G. Darlison, and P. Seeburg:Trends Neurosci. 10, 502–509 (1987).
G. Fasman and W. A. Gilbert:Trends Biochem. Sci. 15, 89–95 (1990).
P. E. Stein, A. Boodhoo, G. J. Tyrrell, J. L. Brunton, and R. J. Read:Nature 355, 748–750 (1992).
J. D. Lear, Z. R. Wasserman, and W. F. DeGrado:Science 240, 1177–1181 (1988).
W. F. DeGrado and J. D. Lear:Biopol. 29, 205–213 (1990).
K. S. Akerfeldt, J. D. Lear, Z. R. Wasserman, L. A. Chung, and W. F. DeGrado:Acc. Chem. Res. 26, 191–197 (1993).
S. Oiki, W. Danho, V. Madison, and M. Montal:Proc. Natl. Acad. Sci. USA 85, 8703–8707 (1988).
S. Oiki, V. Madison, and M. Montal:Proteins 8, 226–236 (1990).
A. Grove, J. M. Tomich, T. Iwamoto, and M. Montal:Prot. Sci. 2, 1918–1930 (1993).
M. O. Montal, T. Iwamoto, J. M. Tomich, and M. Montal:FEBS Letters 320, 261–266 (1993).
M. Oblattmontal, L. K. Buhler, T. Iwamoto, J. M. Tomich, and M. Montal:Journal of Biological Chemistry 268, 14601–14607 (1993).
G. L. Reddy, T. Iwamoto, J. M. Tomich, and M. Montal:J. Biol. Chem. 268, 14608–14615 (1993).
S. J. Weiner, P. A. Kollman, D. A. Case, U. C. Singh, C. Ghio, G. Alagona, S. J. Profeta, and P. Weiner:J. Am. Chem. Soc. 106, 765–784 (1984).
K. Cooper, E. Jakobsson, and P. Wolynes:Prog. Biophys. molec. Biol. 46, 51–96 (1985).
D. G. Levitt:Biophys. J. 59, 271–277 (1991).
D. G. Levitt:Biophys. J. 59, 278–288 (1991).
B. Roux and M. Karplus:Biophys. J. 59, 961–981 (1991).
V. Barcilon, D. P. Chen, and R. S. Eisenberg:SIAM J. Appl. Math. 52, 1405–1425 (1992).
V. Barcilon:SIAM J. Appl. Math. 52, 1391–1404 (1992).
E. Jakobsson:Int. J. Quant. Chem. 25–36 (1993).
S. Bek and E. Jakobsson:Biophys. J. 66, 1028–1038 (1994).
D.J. Adams, T. M. Dwyer, and B. Hille:J. Gen. Physiol. 75, 493–510 (1980).
H. Eyring, R. Lumry, and J. W. Woodbury:Rec. Chem. Prog. 10, 100–114 (1949).
S. Furois-Corbin and A. Pullman:FEBS Letters 252, 63–68 (1989).
S. Furois-Corbin and A. Pullman:Biophys. Chem. 39, 153–159 (1991).
G. Eisenman and O. Alvarez:J. Membrane Biol. 119, 109–132 (1991).
C. Gruber, J. L. Lebowitz, and P. A. Martin:J. Chem. Phys. 75, 944–954 (1981).
S.-W. Chiu, J. A. Novotny, and E. Jakobsson:Biophys. J. 64, 98–109 (1993).
D. P. Chen, V. Barcilon, and R. S. Eisenberg:Biophys. J. 61, 1372–1393 (1992).
F. F. Offner:Biophys. J. 62, 281–283 (1992).
E. Jakobsson:Biophys. J. 64, A201 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
von Kitzing, E. Structure modeling of the acetylcholine receptor channel and related ligand gated channels. Mol Eng 5, 25–43 (1995). https://doi.org/10.1007/BF00999576
Issue Date:
DOI: https://doi.org/10.1007/BF00999576