Abstract
Oligonucleotides got more and more into focus for therapeutic purposes. Administration of such molecules is a challenge, as surviving the bloodstream passage and passing the barrier cell membrane are almost insuperable tasks. Although successful clinical studies have been conducted with naked oligonucleotides, such as antisense agents or siRNA, poor cellular uptake and low cellular persistence reveal the need for adequate carriers.
Delivery of the undamaged oligonucleotide to its site of action has been explored with manifold systems. However, these systems all have one aim: protection of the cargo during the bloodstream passage, facilitation of cellular uptake, and, finally, payload release into the cytosol. Size plays also an important role for the physiological pathway, as particles, if their size is suboptimal, may either clog blood vessels, be removed by the reticuloendothelial system, or undergo rapid renal clearance.
Therefore, research in this field takes advantage of natural nucleic acid encapsulation systems (viruses) or aims at mimicking virus-like features with nonviral carriers. This review focuses on the principles of oligonucleotide encapsulation or packaging with different classes of carrier molecules towards therapeutic use.
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Inaccurate processes on cellular level are the origin of various severe diseases. Depending on the nature of the genetic disorder, two main therapeutic strategies can be pursued. One possibility to adjust the life machinery is the replacement of missing or defective genes by introduction of correspondingly encoding DNA into cells.
Alternatively, inaccurate or overexpressed genes with pathological consequences can be deactivated. This latter strategy has an interesting history. More than 30 years ago, the first steps were made towards knocking down genes by the antisense approach using single-stranded oligonucleotides (Zamecnik and Stephenson 1978), but only 13 years ago, the RNA interference pathway was discovered (Fire et al. 1998). Silencing of target genes may take place by small-interfering RNA (siRNA)-mediated mRNA degradation (Hannon 2002; Meister and Tuschl 2004; Zamore and Haley 2005).
Two main possibilities of utilizing the RNAi pathway for therapeutic purposes exist. One is based on the discovery that extracellularly applied artificial double-stranded siRNAs, 20–24 nucleotides long, as soon as taken up into the cell, can enter the RNAi pathway through cytosolic RISC incorporation (Elbashir et al. 2001). The other approach is based on plasmid-based intranuclear expression of short-hairpin RNA (shRNA) (Brummelkamp et al. 2002), which is processed by the cell after entering the cytosol into the corresponding siRNA. This method leads to stable gene silencing, while artificial siRNA attains only transient knockdown, as it is diluted and lost during cell division.
Another naturally occurring class of double-stranded oligonucleotides, namely, microRNAs (miRNAs), utilize RNA interference for regulation of many targets at the same time (Bartel 2004). Deregulation of microRNA expression in tumors (overexpression of oncomiRs, loss of expression of suppressor miRs) suggests their role as either therapeutic targets or therapeutic agents (Lu et al. 2005; Gregory and Shiekhattar 2005).
However, before acting on intracellular level, oligonucleotides have to reach the target tissue and enter the cells, which is a challenging task. Systemic application involves several risks for “naked” oligonucleotides, such as enzymatic degradation during blood circulation and rapid renal clearance. Another challenge is reaching the target tissue and passing the barrier cell membrane before reaching the site of action. So as to overcome these challenges, accurate protection of the nucleotide cargo by chemical modification and/or packaging into viral or nonviral nanocarriers is mandatory and has been widely studied during the last decades.
This review presents the latest developments of oligonucleotide encapsulation and packaging methods and their potential for therapeutic use.
2 Nucleic Acid Encapsulation and Packaging Methods
Reaching the target tissue and entering cells without loss of activity is, as already mentioned, an insuperable challenge for systemically applied naked oligonucleotides. The size of such particles is a very important issue. Large particles may get stuck in tight blood vessels and lead to tissue hypoxia or even death. Capture and inactivation by the reticuloendothelial system is another undesired outcome. If nanoparticles are too small, like naked oligonucleotides, they may be subjected to rapid renal clearance without having the possibility of systemic circulation sufficient to reach the target tissue.
As the natural ideal nucleic acid carriers, namely, viruses, have a size between 20 and 300 nm, the same range is valid for particles formed with nonviral vectors and the nucleic acid. Carriers for plasmid DNA-based gene delivery have been evaluated for decades now, but unfortunately the requirements for the delivery of oligonucleotides or siRNA are not exactly the same (Scholz and Wagner 2012; Kwok and Hart 2011). Still, since the discovery of RNA interference in C. elegans (Fire et al. 1998) and in mammalian cells (Elbashir et al. 2001), research in this field has tremendously advanced towards therapeutic application in humans.
Already in the 1990s, antisense oligonucleotides were developed for clinical application, and siRNA for local treatment of CMV retinitis was successfully tested in clinical trials (Azad et al. 1993; Crooke 1998; Grillone and Lanz 2001). However, short in vivo half-life and transient transfection hamper the use of naked oligonucleotides. Therefore, packaging methods and delivery strategies have been developed in order to overcome these drawbacks.
3 Viral Vectors for Oligonucleotide Delivery
Viruses are natural carrier systems for the delivery of any kind of nucleic acid optimized in host interactions over very long time. They are programmed by nature to reach the target tissue, enter cells, and set free the nucleic acid for their own replication. Therefore, it seems rightly reasonable that these highly specific natural shuttles, due to their ability to adapt to changing environmental conditions, found application as nucleic acid vectors with very promising therapeutic potential. Different viral vector systems have been evaluated during the past years for oligonucleotide delivery. Replication-defective adeno-associated viruses (AAV) packaging their genome into pure protein capsids, and also lentiviruses (LV), a subclass of retroviruses, containing the nucleic acid/protein capsid within a lipid envelope, showed high efficiency combined with the best safety profiles (Couto and High 2010; Sliva and Schnierle 2010). These vectors are able of transducing both dividing and nondividing cells, thus allowing high gene transfer rates.
For persistent intracellular siRNA activity, a DNA-based method for sustained intracellular siRNA expression was developed (Brummelkamp et al. 2002; Paddison et al. 2002). Plasmid DNA cassettes encoding for the corresponding shRNA are transcribed in the nucleus and further processed in the cytosol, leading to sustained gene knockdown. Thus, in a further step, such shRNA-encoding cassettes were encapsulated in viral vectors for targeted delivery. Beverly Davidson used adeno-associated viruses (AAV) for therapeutic use by shRNA expression (Xia et al. 2004). This strategy was further pursued and evaluated in mouse models, aiming at treatment of various diseases such as prion disease or Huntington’s disease, viral infections like HBV, HCV, and HIV (Couto and High 2010). AAV were also used for the delivery of shRNA for eradication of prostate cancer xenografts (Sun et al. 2010).
Furthermore, Anderson et al. (2007) combined three anti-HIV RNAs in one lentiviral vector. In a xenograft mouse model, by the simultaneous delivery of shRNA, TAR decoy, and anti-CCR5 ribozyme into human CD34+ cells, T cells with maintained functionality but HIV resistance were generated. This threefold combination vector is used at the moment in a clinical trial for AIDS/lymphoma patients (DiGiusto et al. 2010).
Viral vectors can also be used for substituting downregulated suppressor miRNAs in tumors. Kota et al. (2009) have shown that systemic administration of an AAV vector expressing the suppressed miRNA in a mouse liver cancer model dramatically reduced tumor progression.
Although the results achieved with viral vectors in RNAi are very promising, this application for therapeutic purposes is still accompanied by a few challenges. shRNA expressed by viral vectors, for instance, can induce immune responses such as interferon (IFN) induction (Kenworthy et al. 2009; Fish and Kruithof 2004) or toxicity can occur due to oversaturation of the RNAi pathway (Grimm et al. 2006). Another significant drawback is the safety issues related to viral vectors, such as their potential of immune response induction or the risk of insertion mutagenesis. Furthermore, low loading capacity and difficult large-scale production may limit therapeutic application (Tomanin and Scarpa 2004; Devroe and Silver 2004).
4 Synthetic Carriers for Oligonucleotide Delivery
The research work dedicated to development and optimization of nonviral nucleic acid formulations is motivated out of the hope for a virus-like high transfection efficiency with minimal or no side effects. Viral features, though, can be considered and imitated, as viruses represent natural examples of most effective carrier system. Several classes of synthetic carriers have evolved out of this aim. Like in viruses, the formulation of siRNA or other oligonucleotides may follow significantly different rules depending on the chemical (lipid-free or lipid-based) composition of the carrier.
In the following sections, different classes of synthetic formulations used for oligonucleotide encapsulation for therapeutic purpose will be described.
5 Natural Cationic Polymers
Natural cationic polymers such as chitosan, atelocollagen, or protamine have the advantage of being biodegradable and nontoxic. Packaging of the oligonucleotide occurs via electrostatic interactions between the positively charged polymer and the negatively charged backbone of the cargo, thus forming nano-sized polyplexes, usually bearing a positive surface charge. Furthermore, encapsulation of siRNA has already been shown to enable biocompatible and efficient gene silencing.
Atelocollagen-siRNA polyplexes, for instance, were used for tumor growth inhibition in a germ cell tumor xenograft mouse model (Minakuchi et al. 2004). Also, vascular endothelial growth factor inhibition achieved with such polyplexes suppressed tumor angiogenesis and growth (Takei et al. 2004).
The natural, arginine-rich cationic polypeptide protamine has the ability for efficient condensation of nucleic acids. Complexes consisting of a cell-penetrating peptide derived from natural arginine-rich protamine and siRNA suppress tumor growth in a mouse hepatocarcinoma model (Choi et al. 2010) without induction of immunostimulatory effects.
Nanoparticles formed with the natural cationic polysaccharide chitosan, for instance, were shown to mediate effective gene silencing after either intravenous (Pille et al. 2006) or intraperitoneal (Howard et al. 2009) administration while at the same time displaying favorable safety profiles.
Cyclodextrin-based oligocations have also proven their capability of acting as biocompatible and efficient nucleic acid carriers. Targeted siRNA-cyclodextrin nanoparticles, with transferrin as targeting ligand and polyethyleneglycol (PEG) for shielding, have been used in mouse models for treatment of metastatic Ewing’s sarcoma (Hu-Lieskovan et al. 2005). Such nanoparticles were also intravenously administered to patients with solid tumors in a clinical trial (Davis et al. 2010), achieving specific gene inhibition.
6 Synthetic Cationic Polymers
A synthetic polymer-based backbone offers the possibility for a wide spectrum of modifications with regard to optimization for a given cargo. One of the leading synthetic polycation for pDNA delivery is the “gold standard” polyethylenimine (PEI) (Fig. 9.1c). PEI has outstanding features like excellent interaction with nucleic acids, pronounced endosomal buffering capacity, which is important for the endosomal escape, and high accessibility for modification (Boussif et al. 1995; Akinc et al. 2005). Therefore, it seemed reasonable that PEI, either linear or branched, might be useful for oligonucleotide and siRNA delivery. In order to avoid toxicity after systemic administration (Moghimi et al. 2005), various modifications of PEI were investigated, such as PEGylation (modification with polyethylene glycol; PEG) (Tsai et al. 2011; Beyerle et al. 2011), introduction of targeting moieties or lytic domains (Kwon et al. 2008), deacylation (Thomas et al. 2005), alkylation (Fortune et al. 2011), or succinoylation (Zintchenko et al. 2008). PEI modified with stearic acid was used as cationic counterpart for the formation of siRNA polyplexes for induction of tumor apoptosis in a melanoma cell line (Alshamsan et al. 2010).
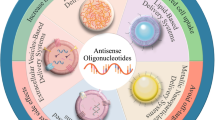
Examples of carriers used for oligonucleotide encapsulation. (a) Adenovirus. (b) Liposome consisting of a bilayer membrane with the lipid tails clustered together. (c) Linear PEI. (d) DOTAP. (e) Exemplified structure of sequence-defined polymers consisting of a cross-linking domain, a succinoyl tetraethylene pentamine unit and a bis (fatty acid) tail
Schaffert et al. (2011b) combined different domains in one carrier, each for fulfilling another task, for delivery of the apoptosis, and for immune response inducing double-stranded RNA polyinosinic:polycytidylic acid, poly(I:C). Branched 25 kDa PEI was modified with PEG as a shielding domain, the tumor cell targeting moiety epidermal growth factor (EGF), and melittin for enhancing endosomal escape. The group could show growth retardation of human A431 xenograft tumors in mice.
The polypeptide polylysine (PLL) was also used as a cationic polymer for siRNA delivery. PLL on its own does not mediate efficient transfection and additionally has been shown by Symonds et al. to induce apoptosis in three different human cell lines (Symonds et al. 2005). Therefore, it was modified by Meyer et al. with different moieties, each responsible for facilitating a step in the delivery pathway, all together resulting in a very efficient delivery construct (Meyer et al. 2008, 2009).
7 Precise and Sequence-Defined Synthetic Carriers
Most of the polymers mentioned above and also others developed up to now have a significant drawback, which is polydispersity of the carrier. This negative feature hampers precise conclusions on structure–activity relationships, as the efficiency or also toxic effects cannot be traced back to one single compound. Also, large-scale GMP production requires identical reproducible batches; polydispersity impedes achieving this aim.
Development of dendritic polymers was a step towards solving this drawback. Different synthetic strategies enable the development of branched three-dimensional molecules with a well-defined structure and a narrow polydispersity range. The options for modification and improvement are very broad and have been widely investigated for gene delivery. Dendritic gene carriers such as polyamidoamine (PAMAM), polypropylenimine (PPI), or dendritic polylysine (PLL) with different surface and internal modifications have been investigated for delivery of antisense nucleotides, siRNA, and DNAzymes. A more detailed overview is given in Ravina et al. (2010).
In contrast to polymers, peptides designed for oligonucleotide and siRNA delivery (Andaloussi et al. 2011; Ezzat et al. 2011; Leng and Mixson 2005) benefit from precise sequence-defined assembly by solid-phase-supported synthesis (SPS). Hartmann and Börner took first advantage of this methodology for the generation of precise, sequence-defined poly(amidoamines) (Hartmann et al. 2006). These compounds were shown to efficiently condense pDNA (Hartmann et al. 2008).
Schaffert et al. (2011a) further developed this strategy by the generation of precise cationic oligo(aminoethane)-based building blocks suitable for SPS, thus enabling fast and parallel synthesis of structural variations. The oligoamino acid building blocks were designed to contain the 1,2-diaminoethane motif (Wagner 2012) which is part of a series of very effective polycationic carriers such as PEI or related degradable carriers (Miyata et al. 2012). In a subsequent publication, an excerpt was given over selected compounds out of a library of more than 300 structures with differing topologies and containing activity-enhancing moieties such as cysteines or fatty acids (Fig. 9.1e). These precise oligomers did not only show efficient transfection for pDNA, reaching levels equivalent to PEI, but also knockdown of the target gene down to approximately 10 % with very good maintenance of cell viability and low in vivo cytotoxicity (Schaffert et al. 2011c). Structure–activity relationships of these carriers with siRNA were further evaluated in vitro and in vivo (Frohlich et al. 2012). Apart from linear topologies, also branched structures such as four-arms and the influence of various different building blocks were tested (Salcher et al. 2012). Also, novel constructs generated by SPS containing of PEG moieties and targeting ligands such as folic acid could efficiently complex and deliver siRNA and knock down the target genes (Dohmen et al. 2012; Martin et al. 2012).
8 Cationic Lipids and Liposomes
Synthetic lipids containing a polar head group and a lipid tail have the ability to form so-called liposomes (Fig. 9.1b). Hydrophilic structures like oligonucleotides can be encapsulated in the aqueous core of such liposomes. Encapsulation may take advantage of inclusion of cationic lipids in the bilayer. Electrostatic interactions between the cationic lipids and the negatively charged nucleic acid strongly enhance the encapsulation efficiency. Alternatively, cationic liposomes may also form complexes (lipoplexes) with nucleic acid without encapsulation into the interior of lipid bilayer membranes. In both types of the formed particles, the lipidic structures can provide oligonucleotide cargo protection after systemic delivery. Moreover, a crucial advantage is the interaction of the cationic lipid with anionic lipids in the cellular and endosomal membrane, thus facilitating cell entry and endosomal escape as basis for cargo release into the cytosol.
The cationic lipid DOTMA (N-[1-(2,3-Dioleyloxy)propyl]-N,N,N-trimethylammoniumchlorid chloride) was the first synthetic lipid used for gene delivery to mammalian cells and was presented in 1989 (Felgner and Ringold 1989), followed by the delivery of antisense oligonucleotides and ribozymes (Akhtar et al. 2000; Hughes et al. 2001). Since then, these promising vectors were further improved and evaluated for oligonucleotide delivery. The cationic lipid DOTAP (Fig. 9.1d), for instance, was used for siRNA encapsulation and subsequent melanoma inhibition (Tran et al. 2008).
Cationic lipids were also commercialized, such as Lipofectamine 2000 (Invitrogen) and i-FECT (Neuromics), and widely used for siRNA encapsulation and transfection (Yokota et al. 2007; Kumar et al. 2006). Additionally, Lipofectamine was used for inhibiting HIV-I expression by transfection of encapsulated locked nucleic acid (LNA)-modified antisense oligonucleotides and DNAzymes (Jakobsen et al. 2007).
Particle stability is also a crucial issue concerning systemic delivery. Hence, stable nucleic acid lipid particles (SNALPs) got more and more into focus. SNALPs are PEG-lipid-coated liposomes consisting of different cationic and fusogenic lipids. The coating provides particle stability during formulation and also hampers undesired interactions with blood components.
In vivo studies conducted by Morrissey et al. (2005) showed inhibition of HBV replication by the administration of anti-HBV siRNA-SNALP complexes. Furthermore, systemic delivery of siRNA-SNALP formulation targeting apolipoprotein B (ApoB) in nonhuman primates led to efficient protein reduction lasting for 11 days after injection (Zimmermann et al. 2006; Frank-Kamenetsky et al. 2008). The SNALP formulation contained the lipids 3-N-[(methoxypoly(ethylene glycol))carbamoyl]-1,2-dimyristyloxy-propylamine (PEG-C-DMA), 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane (DLinDMA), and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and cholesterol at specified ratios.
Highly effective guinea pig postexposure protection from the lethal Zaire Ebola virus was shown by treatment with SNALP-encapsulated siRNA (Geisbert et al. 2006). In a subsequent study, a combination of SNALP-embedded siRNAs targeting the expression of three viral proteins was used for preventing macaques from lethal hemorrhagic fever induced by the Ebola virus (Geisbert et al. 2010). Due to their high efficiency and positive safety profile, SNALPs are currently being evaluated in clinical trials (Burnett and Rossi 2012).
Semple et al. used the ionizable cationic lipid 1,2-dilinoleyloxy-3-dimethylaminopropane (DLinDMA) as a base substance for the design of a library of 1,200 lipid nanoparticle compounds. These were screened for siRNA delivery, and the best performer was used for SNALP formulation and characterization. Gene silencing was tested in rodents and nonhuman primates with a successful outcome at low-dose application (Semple et al. 2010).
PEGylation of cationic lipoplexes (Sonoke et al. 2008) leads to increasing plasma concentration of the lipoplex and also to a higher accumulation in tumor. However, PEGylation also hampers cellular uptake and endosomal escape. Hatakeyama and colleagues therefore introduced between PEG and their multifunctional envelope-type nano-device (MEND) an enzymatically cleavable bond which should release the PEG moiety and thus facilitate cellular uptake. The molecule was further modified with the pH-sensitive fusogenic peptide GALA for enhanced endosomal escape, and encapsulated siRNA showed efficient knockdown after intratumoral delivery compared to unmodified MEND (Hatakeyama et al. 2009).
Toxicity, though, is an issue with cationic lipid particles, as has been reported both in vitro and in vivo (Ma et al. 2005; Lv et al. 2006; Akhtar and Benter 2007). The use of neutral lipids for encapsulation and delivery of siRNA should help to avoid toxicity associated with positively charged polymers. Peer et al. (2008) developed liposomes (neutral) coated with hyaluronan for stabilization and covalently attached monoclonal antibody on the surface. Condensed CyD1-siRNA was loaded into these particles, and efficient gene silencing in an intestinal inflammation murine model after IV application could be shown. In another study, siRNA targeting oncoprotein EphA2 encapsulated into neutral liposome 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) (Landen et al. 2005) led to inhibited tumor growth in mice bearing ovarian tumors.
In order to circumvent undesired side effects caused by synthetic lipid structures, natural lipid analogs such as cardiolipin were evaluated for siRNA delivery (Chien et al. 2005; Pal et al. 2005).
A large library of lipid-like materials, so-called lipidoids, was developed and tested for the ability to transfect siRNA in vitro (Akinc et al. 2008), thus finding a leading structure for in vivo formulations. Further studies with this leading structure encapsulating siRNA showed efficient and sustained gene silencing in lung and liver targets in mice, rats, and cynomolgus monkeys (Akinc et al. 2008; Frank-Kamenetsky et al. 2008; Epiphanio et al. 2008).
Saw and colleagues loaded oligodeoxynucleotides complexed with cell-penetrating peptide (CPP) into CPP surface-modified liposomes (anionic) for successfully sensitizing glioblastoma cells to the effects of chemotherapeutic agent paclitaxel (Saw et al. 2010).
Another class of lipid-based vectors are solid lipid nanoparticles (SLN) consisting of natural or synthesized lipids, resulting in biocompatible and biodegradable lipid systems (zur Muhlen et al. 1998) which were primarily used for gene delivery (del Pozo-Rodriguez et al. 2007; Pan et al. 2009; Choi et al. 2008). SLN were also used for encapsulation of anti-microRNA oligonucleotides (AMO) in order to decrease human lung cancer cell functions related to microRNA-21 (Shi et al. 2012).
9 Conclusion and Prospects
Oligonucleotides possess a great therapeutic potential, as their gene sequence specificity can be exploited for finding the best strategy for disease treatment at its genetic origin. Delivery of oligonucleotides for therapeutic purposes has made vast progress during the last decades. Different strategies have been evaluated, all pursuing one aim—treating humans suffering of severe disorders. Nevertheless, appropriated shuttles have to be found for target cell transfection. Suitable encapsulation protects the cargo during blood circulation, mediates cellular uptake, and, depending of the payload nature, can lead to a sustained effect on the target gene.
Various encapsulation methods offer several options, all still bearing advantages but also drawbacks, though. Viruses, for instance, are the perfect nucleic acid carriers designed by nature. Thus, their outstanding properties concerning cargo protection and delivery were widely exploited for therapeutic purposes. Nevertheless, despite the highly promising results achieved with viral vectors, safety issues still hamper establishment as therapeutic agents. Therefore, nonviral oligonucleotide encapsulation methods and delivery strategies are getting more and more into focus. Either synthetic or natural polymers, cationic or neutral lipids have been evaluated for their therapeutic potential. The type of delivery system also has to be well chosen, though, as cationic lipids and polymers may have an impact on siRNA-caused immune stimulation and undesired off-target effects (Akhtar and Benter 2007; Hollins et al. 2007; Omidi et al. 2003, 2005). Even so, other features such as a positive safety profile, low production costs, and high loading capacity have led such carrier systems towards clinical trials, thus marking the first steps towards curing genetically based disorders by tackling the problem at its origin.
References
Akhtar S, Benter I (2007) Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev 59(2–3):164–182. doi:10.1016/j.addr.2007.03.010
Akhtar S, Hughes MD, Khan A, Bibby M, Hussain M, Nawaz Q, Double J, Sayyed P (2000) The delivery of antisense therapeutics. Adv Drug Deliv Rev 44(1):3–21
Akinc A, Thomas M, Klibanov AM, Langer R (2005) Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med 7(5):657–663
Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG (2008) A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 26(5):561–569. doi:10.1038/nbt1402
Alshamsan A, Hamdy S, Samuel J, El-Kadi AO, Lavasanifar A, Uludag H (2010) The induction of tumor apoptosis in B16 melanoma following STAT3 siRNA delivery with a lipid-substituted polyethylenimine. Biomaterials 31(6):1420–1428. doi:10.1016/j.biomaterials.2009.11.003
Andaloussi SE, Lehto T, Mager I, Rosenthal-Aizman K, Oprea II, Simonson OE, Sork H, Ezzat K, Copolovici DM, Kurrikoff K, Viola JR, Zaghloul EM, Sillard R, Johansson HJ, Said Hassane F, Guterstam P, Suhorutsenko J, Moreno PM, Oskolkov N, Halldin J, Tedebark U, Metspalu A, Lebleu B, Lehtio J, Smith CI, Langel U (2011) Design of a peptide-based vector, PepFect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic Acids Res 39(9):3972–3987. doi:10.1093/nar/gkq1299
Anderson J, Li MJ, Palmer B, Remling L, Li S, Yam P, Yee JK, Rossi J, Zaia J, Akkina R (2007) Safety and efficacy of a lentiviral vector containing three anti-HIV genes – CCR5 ribozyme, tat-rev siRNA, and TAR decoy – in SCID-hu mouse-derived T cells. Mol Ther 15(6):1182–1188. doi:10.1038/sj.mt.6300157, 6300157 [pii]
Azad RF, Driver VB, Tanaka K, Crooke RM, Anderson KP (1993) Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob Agents Chemother 37(9):1945–1954
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Beyerle A, Braun A, Merkel O, Koch F, Kissel T, Stoeger T (2011) Comparative in vivo study of poly(ethylene imine)/siRNA complexes for pulmonary delivery in mice. J Control Release 151(1):51–56. doi:10.1016/j.jconrel.2010.12.017
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA 92(16):7297–7301
Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296(5567):550–553. doi:10.1126/science.10689991068999 [pii]
Burnett JC, Rossi JJ (2012) RNA-based therapeutics: current progress and future prospects. Chem Biol 19(1):60–71. doi:10.1016/j.chembiol.2011.12.008
Chien PY, Wang J, Carbonaro D, Lei S, Miller B, Sheikh S, Ali SM, Ahmad MU, Ahmad I (2005) Novel cationic cardiolipin analogue-based liposome for efficient DNA and small interfering RNA delivery in vitro and in vivo. Cancer Gene Ther 12(3):321–328. doi:10.1038/sj.cgt.7700793
Choi SH, Jin SE, Lee MK, Lim SJ, Park JS, Kim BG, Ahn WS, Kim CK (2008) Novel cationic solid lipid nanoparticles enhanced p53 gene transfer to lung cancer cells. Eur J Pharm Biopharm 68(3):545–554. doi:10.1016/j.ejpb.2007.07.011
Choi YS, Lee JY, Suh JS, Kwon YM, Lee SJ, Chung JK, Lee DS, Yang VC, Chung CP, Park YJ (2010) The systemic delivery of siRNAs by a cell penetrating peptide, low molecular weight protamine. Biomaterials 31(6):1429–1443. doi:10.1016/j.biomaterials.2009.11.001, S0142-9612(09)01198-3 [pii]
Couto LB, High KA (2010) Viral vector-mediated RNA interference. Curr Opin Pharmacol 10(5):534–542. doi:10.1016/j.coph.2010.06.007
Crooke ST (1998) Vitravene – another piece in the mosaic. Antisense Nucleic Acid Drug Dev 8(4):vii–viii
Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A (2010) Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464(7291):1067–1070. doi:10.1038/nature08956
del Pozo-Rodriguez A, Delgado D, Solinis MA, Gascon AR, Pedraz JL (2007) Solid lipid nanoparticles: formulation factors affecting cell transfection capacity. Int J Pharm 339(1–2):261–268. doi:10.1016/j.ijpharm.2007.03.015
Devroe E, Silver PA (2004) Therapeutic potential of retroviral RNAi vectors. Expert Opin Biol Ther 4(3):319–327. doi:10.1517/14712598.4.3.319
DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A, Mi S, Yam P, Stinson S, Kalos M, Alvarnas J, Lacey SF, Yee JK, Li M, Couture L, Hsu D, Forman SJ, Rossi JJ, Zaia JA (2010) RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med 2(36):36ra43. doi:10.1126/scitranslmed.3000931
Dohmen C, Edinger D, Frohlich T, Schreiner L, Lachelt U, Troiber C, Radler J, Hadwiger P, Vornlocher HP, Wagner E (2012) Nanosized multifunctional polyplexes for receptor-mediated SiRNA delivery. ACS Nano 6(6):5198–5208
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411(6836):494–498
Epiphanio S, Mikolajczak SA, Goncalves LA, Pamplona A, Portugal S, Albuquerque S, Goldberg M, Rebelo S, Anderson DG, Akinc A, Vornlocher HP, Kappe SH, Soares MP, Mota MM (2008) Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe 3(5):331–338. doi:10.1016/j.chom.2008.04.003
Ezzat K, El Andaloussi S, Zaghloul EM, Lehto T, Lindberg S, Moreno PM, Viola JR, Magdy T, Abdo R, Guterstam P, Sillard R, Hammond SM, Wood MJ, Arzumanov AA, Gait MJ, Smith CI, Hallbrink M, Langel U (2011) PepFect 14, a novel cell-penetrating peptide for oligonucleotide delivery in solution and as solid formulation. Nucleic Acids Res 39(12):5284–5298. doi:10.1093/nar/gkr072, gkr072 [pii]
Felgner PL, Ringold GM (1989) Cationic liposome-mediated transfection. Nature 337(6205):387–388. doi:10.1038/337387a0
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391(6669):806–811
Fish RJ, Kruithof EK (2004) Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors. BMC Mol Biol 5:9. doi:10.1186/1471-2199-5-9
Fortune JA, Novobrantseva TI, Klibanov AM (2011) Highly effective gene transfection in vivo by alkylated polyethylenimine. J Drug Deliv 2011:204058. doi:10.1155/2011/204058
Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, Butler D, Charisse K, Dorkin R, Fan Y, Gamba-Vitalo C, Hadwiger P, Jayaraman M, John M, Jayaprakash KN, Maier M, Nechev L, Rajeev KG, Read T, Rohl I, Soutschek J, Tan P, Wong J, Wang G, Zimmermann T, de Fougerolles A, Vornlocher HP, Langer R, Anderson DG, Manoharan M, Koteliansky V, Horton JD, Fitzgerald K (2008) Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA 105(33):11915–11920. doi:10.1073/pnas.0805434105, 0805434105 [pii]
Frohlich T, Edinger D, Klager R, Troiber C, Salcher E, Badgujar N, Martin I, Schaffert D, Cengizeroglu A, Hadwiger P, Vornlocher HP, Wagner E (2012) Structure-activity relationships of siRNA carriers based on sequence-defined oligo (ethane amino) amides. J Control Release 160(3):532–541. doi:10.1016/j.jconrel.2012.03.018
Geisbert TW, Hensley LE, Kagan E, Yu EZ, Geisbert JB, Daddario-DiCaprio K, Fritz EA, Jahrling PB, McClintock K, Phelps JR, Lee AC, Judge A, Jeffs LB, MacLachlan I (2006) Postexposure protection of guinea pigs against a lethal ebola virus challenge is conferred by RNA interference. J Infect Dis 193(12):1650–1657. doi:10.1086/504267
Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, Maclachlan I (2010) Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet 375(9729):1896–1905. doi:10.1016/S0140-6736(10)60357-1
Gregory RI, Shiekhattar R (2005) MicroRNA biogenesis and cancer. Cancer Res 65(9):3509–3512. doi:10.1158/0008-5472.CAN-05-0298
Grillone LR, Lanz R (2001) Fomivirsen. Drugs Today (Barc) 37(4):245–255
Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA (2006) Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441(7092):537–541. doi:10.1038/nature04791
Hannon GJ (2002) RNA interference. Nature 418(6894):244–251. doi:10.1038/418244a
Hartmann L, Krause E, Antonietti M, Borner HG (2006) Solid-phase supported polymer synthesis of sequence-defined, multifunctional poly(amidoamines). Biomacromolecules 7(4):1239–1244
Hartmann L, Hafele S, Peschka-Suss R, Antonietti M, Borner HG (2008) Tailor-made poly(amidoamine)s for controlled complexation and condensation of DNA. Chemistry 14(7):2025–2033
Hatakeyama H, Ito E, Akita H, Oishi M, Nagasaki Y, Futaki S, Harashima H (2009) A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release 139(2):127–132. doi:10.1016/j.jconrel.2009.06.008, S0168-3659(09)00418-0 [pii]
Hollins AJ, Omidi Y, Benter IF, Akhtar S (2007) Toxicogenomics of drug delivery systems: exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. J Drug Target 15(1):83–88. doi:10.1080/10611860601151860
Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, Kjems J (2009) Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther 17(1):162–168. doi:10.1038/mt.2008.220
Hughes MD, Hussain M, Nawaz Q, Sayyed P, Akhtar S (2001) The cellular delivery of antisense oligonucleotides and ribozymes. Drug Discov Today 6(6):303–315
Hu-Lieskovan S, Heidel JD, Bartlett DW, Davis ME, Triche TJ (2005) Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing’s sarcoma. Cancer Res 65(19):8984–8992. doi:10.1158/0008-5472.CAN-05-0565
Jakobsen MR, Haasnoot J, Wengel J, Berkhout B, Kjems J (2007) Efficient inhibition of HIV-1 expression by LNA modified antisense oligonucleotides and DNAzymes targeted to functionally selected binding sites. Retrovirology 4:29. doi:10.1186/1742-4690-4-29
Kenworthy R, Lambert D, Yang F, Wang N, Chen Z, Zhu H, Zhu F, Liu C, Li K, Tang H (2009) Short-hairpin RNAs delivered by lentiviral vector transduction trigger RIG-I-mediated IFN activation. Nucleic Acids Res 37(19):6587–6599. doi:10.1093/nar/gkp714, gkp714 [pii]
Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT (2009) Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 137(6):1005–1017. doi:10.1016/j.cell.2009.04.021
Kumar P, Lee SK, Shankar P, Manjunath N (2006) A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med 3(4):e96. doi:10.1371/journal.pmed.0030096
Kwok A, Hart SL (2011) Comparative structural and functional studies of nanoparticle formulations for DNA and siRNA delivery. Nanomedicine 7(2):210–219. doi:10.1016/j.nano.2010.07.005, S1549-9634(10)00239-X [pii]
Kwon EJ, Bergen JM, Pun SH (2008) Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug Chem 19(4):920–927
Landen CN Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK (2005) Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res 65(15):6910–6918. doi:10.1158/0008-5472.CAN-05-0530
Leng Q, Mixson AJ (2005) Small interfering RNA targeting Raf-1 inhibits tumor growth in vitro and in vivo. Cancer Gene Ther 12(8):682–690
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR (2005) MicroRNA expression profiles classify human cancers. Nature 435(7043):834–838. doi:10.1038/nature03702
Lv H, Zhang S, Wang B, Cui S, Yan J (2006) Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release 114(1):100–109. doi:10.1016/j.jconrel.2006.04.014
Ma Z, Li J, He F, Wilson A, Pitt B, Li S (2005) Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem Biophys Res Commun 330(3):755–759. doi:10.1016/j.bbrc.2005.03.041
Martin I, Dohmen C, Mas-Moruno C, Troiber C, Kos P, Schaffert D, Lachelt U, Teixido M, Gunther M, Kessler H, Giralt E, Wagner E (2012) Solid-phase-assisted synthesis of targeting peptide-PEG-oligo(ethane amino)amides for receptor-mediated gene delivery. Org Biomol Chem 10(16):3258–3268. doi:10.1039/c2ob06907e
Meister G, Tuschl T (2004) Mechanisms of gene silencing by double-stranded RNA. Nature 431(7006):343–349. doi:10.1038/nature02873
Meyer M, Philipp A, Oskuee R, Schmidt C, Wagner E (2008) Breathing life into polycations: functionalization with pH-responsive endosomolytic peptides and polyethylene glycol enables siRNA delivery. J Am Chem Soc 130(11):3272–3273
Meyer M, Dohmen C, Philipp A, Kiener D, Maiwald G, Scheu C, Ogris M, Wagner E (2009) Synthesis and biological evaluation of a bioresponsive and endosomolytic siRNA-polymer conjugate. Mol Pharm 6(3):752–762. doi:10.1021/mp9000124
Minakuchi Y, Takeshita F, Kosaka N, Sasaki H, Yamamoto Y, Kouno M, Honma K, Nagahara S, Hanai K, Sano A, Kato T, Terada M, Ochiya T (2004) Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Res 32(13):e109. doi:10.1093/nar/gnh093
Miyata K, Nishiyama N, Kataoka K (2012) Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem Soc Rev 41(7):2562–2574
Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A (2005) A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther 11(6):990–995
Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B (2005) Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol 23(8):1002–1007. doi:10.1038/nbt1122
Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S (2003) Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target 11(6):311–323. doi:10.1080/10611860310001636908
Omidi Y, Hollins AJ, Drayton RM, Akhtar S (2005) Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target 13(7):431–443. doi:10.1080/10611860500418881
Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS (2002) Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 16(8):948–958. doi:10.1101/gad.981002
Pal A, Ahmad A, Khan S, Sakabe I, Zhang C, Kasid UN, Ahmad I (2005) Systemic delivery of RafsiRNA using cationic cardiolipin liposomes silences Raf-1 expression and inhibits tumor growth in xenograft model of human prostate cancer. Int J Oncol 26(4):1087–1091
Pan X, Chen L, Liu S, Yang X, Gao JX, Lee RJ (2009) Antitumor activity of G3139 lipid nanoparticles (LNPs). Mol Pharm 6(1):211–220. doi:10.1021/mp800146j
Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M (2008) Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science 319(5863):627–630
Pille JY, Li H, Blot E, Bertrand JR, Pritchard LL, Opolon P, Maksimenko A, Lu H, Vannier JP, Soria J, Malvy C, Soria C (2006) Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: safety and efficacy in xenografted aggressive breast cancer. Hum Gene Ther 17(10):1019–1026. doi:10.1089/hum.2006.17.1019
Ravina M, Paolicelli P, Seijo B, Sanchez A (2010) Knocking down gene expression with dendritic vectors. Mini Rev Med Chem 10(1):73–86
Salcher EE, Kos P, Frohlich T, Badgujar N, Scheible M, Wagner E (2012) Sequence-defined four-arm oligo(ethanamino)amides for pDNA and siRNA delivery: impact of building blocks on efficacy. J Control Release 164(3):380–386
Saw PE, Ko YT, Jon S (2010) Efficient liposomal nanocarrier-mediated oligodeoxynucleotide delivery involving dual use of a cell-penetrating peptide as a packaging and intracellular delivery agent. Macromol Rapid commun 31(13):1155–1162. doi:10.1002/marc.200900861
Schaffert D, Badgujar N, Wagner E (2011a) Novel Fmoc-polyamino acids for solid-phase synthesis of defined polyamidoamines. Org Lett 13(7):1586–1589. doi:10.1021/ol200381z
Schaffert D, Kiss M, Rodl W, Shir A, Levitzki A, Ogris M, Wagner E (2011b) Poly(I:C)-mediated tumor growth suppression in EGF-receptor overexpressing tumors using EGF-polyethylene glycol-linear polyethylenimine as carrier. Pharm Res 28(4):731–741. doi:10.1007/s11095-010-0225-4
Schaffert D, Troiber C, Salcher EE, Frohlich T, Martin I, Badgujar N, Dohmen C, Edinger D, Klager R, Maiwald G, Farkasova K, Seeber S, Jahn-Hofmann K, Hadwiger P, Wagner E (2011c) Solid-phase synthesis of sequence-defined T-, i-, and U-shape polymers for pDNA and siRNA delivery. Angew Chem Int Ed Engl 50(38):8986–8989. doi:10.1002/anie.201102165
Scholz C, Wagner E (2012) Therapeutic plasmid DNA versus siRNA delivery: common and different tasks for synthetic carriers. J Control Release 161(2):554–565. doi:10.1016/j.jconrel.2011.11.014, S0168-3659(11)01044-3 [pii]
Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ (2010) Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 28(2):172–176. doi:10.1038/nbt.1602
Shi SJ, Zhong ZR, Liu J, Zhang ZR, Sun X, Gong T (2012) Solid lipid nanoparticles loaded with anti-microRNA oligonucleotides (AMOs) for suppression of microRNA-21 functions in human lung cancer cells. Pharm Res 29(1):97–109. doi:10.1007/s11095-011-0514-6
Sliva K, Schnierle BS (2010) Selective gene silencing by viral delivery of short hairpin RNA. Virol J 7:248. doi:10.1186/1743-422X-7-248
Sonoke S, Ueda T, Fujiwara K, Sato Y, Takagaki K, Hirabayashi K, Ohgi T, Yano J (2008) Tumor regression in mice by delivery of Bcl-2 small interfering RNA with pegylated cationic liposomes. Cancer Res 68(21):8843–8851. doi:10.1158/0008-5472.CAN-08-0127, 68/21/8843 [pii]
Sun A, Tang J, Terranova PF, Zhang X, Thrasher JB, Li B (2010) Adeno-associated virus-delivered short hairpin-structured RNA for androgen receptor gene silencing induces tumor eradication of prostate cancer xenografts in nude mice: a preclinical study. Int J Cancer 126(3):764–774. doi:10.1002/ijc.24778
Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A, Moghimi SM (2005) Low and high molecular weight poly(L-lysine)s/poly(L-lysine)-DNA complexes initiate mitochondrial-mediated apoptosis differently. FEBS Lett 579(27):6191–6198. doi:10.1016/j.febslet.2005.09.092, S0014-5793(05)01237-8 [pii]
Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T (2004) A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res 64(10):3365–3370. doi:10.1158/0008-5472.CAN-03-2682
Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM (2005) Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci USA 102(16):5679–5684. doi:10.1073/pnas.0502067102, 0502067102 [pii]
Tomanin R, Scarpa M (2004) Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr Gene Ther 4(4):357–372
Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, Smith NB, Robertson GP (2008) Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res 68(18):7638–7649. doi:10.1158/0008-5472.CAN-07-6614
Tsai LR, Chen MH, Chien CT, Chen MK, Lin FS, Lin KM, Hwu YK, Yang CS, Lin SY (2011) A single-monomer derived linear-like PEI-co-PEG for siRNA delivery and silencing. Biomaterials 32(14):3647–3653. doi:10.1016/j.biomaterials.2011.01.059
Wagner E (2012) Polymers for siRNA delivery: inspired by viruses to be targeted, dynamic, and precise. Acc Chem Res 45(7):1005–1013. doi:10.1021/ar2002232
Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, Paulson HL, Yang L, Kotin RM, Davidson BL (2004) RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 10(8):816–820. doi:10.1038/nm1076
Yokota T, Iijima S, Kubodera T, Ishii K, Katakai Y, Ageyama N, Chen Y, Lee YJ, Unno T, Nishina K, Iwasaki Y, Maki N, Mizusawa H, Akari H (2007) Efficient regulation of viral replication by siRNA in a non-human primate surrogate model for hepatitis C. Biochem Biophys Res Commun 361(2):294–300. doi:10.1016/j.bbrc.2007.06.182
Zamecnik PC, Stephenson ML (1978) Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci USA 75(1):280–284
Zamore PD, Haley B (2005) Ribo-gnome: the big world of small RNAs. Science 309(5740):1519–1524. doi:10.1126/science.1111444
Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I (2006) RNAi-mediated gene silencing in non-human primates. Nature 441(7089):111–114. doi:10.1038/nature04688
Zintchenko A, Philipp A, Dehshahri A, Wagner E (2008) Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjug Chem 19(7):1448–1455. doi:10.1021/bc800065f
zur Muhlen A, Schwarz C, Mehnert W (1998) Solid lipid nanoparticles (SLN) for controlled drug delivery – drug release and release mechanism. Eur J Pharm Biopharm 45(2):149–155
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Salcher, E.E., Wagner, E. (2014). Nano-encapsulation of Oligonucleotides for Therapeutic Use. In: Kjems, J., Ferapontova, E., Gothelf, K. (eds) Nucleic Acid Nanotechnology. Nucleic Acids and Molecular Biology, vol 29. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-38815-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-38815-6_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-38814-9
Online ISBN: 978-3-642-38815-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)