Abstract
Radiation therapy has evolved into an integral modality in the treatment of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). As technology has evolved, stereotactic body radiotherapy (SBRT) can now be used to achieve excellent local control in patients with early stage NSCLC who are non-operative candidates. Randomized trials are now comparing SBRT to surgery in early stage NSCLC. Chemoradiotherapy (CRT) is used as definitive therapy in the majority of cases of locally advanced NSCLC and can be used as neoadjuvant or adjuvant treatment to surgery in select cases. Most patients with SCLC will receive radiotherapy to the brain to treat brain metastases or prevent brain metastases, and patient with SCLC receive thoracic radiotherapy. In metastatic lung cancer, radiotherapy is used for palliation of brain metastases, spinal cord compression, superior vena cava syndrome, bony pain, hemoptysis, and airway obstruction. As treatment techniques continue to evolve, radiotherapy alone or in combination with new targeted therapies will continue to play an important role in the management of patients with lung cancer while reducing toxicity.
Similar content being viewed by others
Keywords
- Brain Metastasis
- Small Cell Lung Cancer
- Stereotactic Body Radiotherapy
- Radiation Pneumonitis
- Biological Effective Dose
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
External beam radiation therapy (EBRT) is an important component in the management of lung cancer. Radiotherapy would be required at some point in the treatment of small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) in 50–60 % of patients[1]. Technical advances made in radiation planning and delivery such as 3D conformal radiotherapy (3DCRT), intensity modulated radiotherapy (IMRT), image-guided radiotherapy (IGRT), and stereotactic body radiotherapy (SBRT) have transformed the role of RT in the management of lung cancer, particularly in the last decade. RT is used as definitive therapy in medically inoperable NSCLC (3DCRT, SBRT), as multimodality therapy in locally advanced (stage III) NSCLC and limited stage SCLC, neoadjuvant and adjuvant in stage IIIA NSCLC, palliative treatment of metastasis in stage IV lung cancer, and for prophylactic cranial irradiation (PCI) in both SCLC and NSCLC [2]. Modern techniques of accurate target delineation and normal tissue avoidance have significantly improved chances of loco-regional control of disease as well as reduced normal tissue toxicity.
Definitive Radiotherapy in Medically Inoperable NSCLC
In patients with early stage (I and II) NSCLC who are technically resectable at presentation, lobectomy or pneumonectomy and pathologic mediastinal nodal staging offer the best overall survival. Based on data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute, 30 % of patients with localized NSCLC did not undergo surgical treatment. High rate of comorbid medical illness, poor baseline pulmonary function, advanced age, poor performance status, and patient decision are the most common reasons among this patient population [3]. These patients are treated with definitive RT and more recently, selected patients are treated with SBRT. Five-year survival rates in surgically treated stage IA, IB, and II NSCLC reach 80 %, 50 %, and 35 %, respectively. Five-year survival with conventional RT in this group ranges from 10 to 42 %. Local recurrence and distant failure dominate the causes of failure [4]. Isolated nodal failures are uncommon.
RT dose escalation has been evaluated by several investigators. The University of Michigan lung cancer dose escalation study in stage I NSCLC patients concluded that doses of radiation of 92.4 and 102.9 Gy can be delivered safely to limited lung volumes with minimal toxicity and 2- and 3-year freedom from local progression, overall survival and cause-specific survival rates of 82 % and 68 %, 54 % and 33 %, and 76 % and 48 %, respectively [5]. Urbanic et al. reported 37 % local failure rate for medically inoperable lung cancer patients treated with 3DCRT to 80.5 Gy in 7 weeks [6]. RTOG conducted a phase I–II dose escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small cell lung carcinoma. For patients receiving RT alone or radiation following induction chemotherapy, data from RTOG 9311 established that doses of 83.8 Gy using three-dimensional conformal RT techniques were tolerable, with excess mortality observed at 90.3 Gy. Elective nodal failure occurred in less than 10 % of patients [7].
Stereotactic Body Radiotherapy
Recent developments in IGRT are ushering in a new era of radiotherapy for lung cancer. Positron emission tomography-computed tomography (PET-CT) has been shown to improve targeting accuracy in 25–50 % of cases. Daily on-board imaging reduces treatment setup uncertainty and provides information about daily organ motion and variations in anatomy. Image-guided stereotactic radiotherapy can achieve local control rates exceeding 90 % through the use of focused, hypofractionated, highly biologically effective doses (Figs. 12.1 and 12.2) [8]. Four-dimensional CT scans and abdominal compression are frequently used to account for lung motion and reduce superior to inferior tumor excursion which is critical in treating tight radiation fields adequately [9].
SBRT plan delivering 50 Gy in 5 fractions to a cT1aN0M0 NSCLC of the left upper lobe. A 4D CT was done at the time of simulation and the tumor contoured at all phases of the breathing cycle to generate an internal target volume (ITV). The treatment was delivered free breathing with prescription dose being delivered to the ITV with daily image guidance with cone beam CT (CBCT)
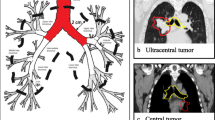
Timmerman et al. published results of a phase I study in medically inoperable stage I NSCLC treated with extracranial stereotactic radioablation. Patients with comorbid medical problems that precluded thoracotomy and with clinically staged T1 or T2 (tumor size 7 cm or less) N0M0 biopsy confirmed NSCLC were included. Patients with T1 vs. T2 tumors underwent independent dose escalations. Both T-stage groups ultimately reached and tolerated 20 Gy per fraction for 3 fractions (total: 60 Gy) [10]. Treatments were completed in 12 days. A phase II study by the same group included 70 T1 and T2 medically inoperable NSCLC patients [11]. Kaplan–Meier local control at 2 years was 95 %. Median overall survival was 32.6 months and 2-year overall survival was 54.7 %. Grade 3–5 toxicity occurred in a total of 14 patients. Patients treated for tumors in the peripheral lung had 2-year freedom from severe toxicity of 83 % compared with only 54 % for patients with central tumors. An important observation from this study was that SBRT of centrally located lesions is associated with increased risk of toxicity and that appropriate patient selection is very important (Fig. 12.3).
Coronal CT image with the proximal bronchial tree (carina, right and left main bronchi, right and left upper lobe bronchi, intermedius bronchus, right middle lobe bronchus, lingular bronchus, and right and left lower lobe bronchi all contoured in red) with a 2 cm expansion (in green) to produce the zone of the proximal bronchial tree. Bronchioles distal to the proximal bronchial tree are contoured in blue. The contours are shown in 3D to the right. Tumors in the zone of the proximal bronchial tree are at risk for increased toxicity when treated with SBRT to large fractional doses (i.e., 60 Gy in 3 fractions)
Onishi and colleagues published results from the Japanese multi-institutional study of hypofractionated stereotactic radiotherapy (HypoFXSRT) for 257 patients with stage I NSCLC [12]. A total dose of 18–75 Gy at the isocenter was administered in 1–22 fractions. The median calculated biological effective dose (BED) was 111 Gy (range: 57–180 Gy). At a median follow-up of 38 months, local recurrence rate was 8.4 % for a BED of 100 Gy or more compared with 42.9 % for less than 100 Gy (p < 0.001). The 5-year overall survival rate of medically operable patients was 70.8 % among those treated with a BED of 100 Gy or more compared with 30.2 % among those treated with less than 100 Gy (p < 0.05). BED is a function of total dose and RT fraction size for a given α/β ratio. Early results of hypofractionated SBRT are promising with limited acute toxicity in selected T1 and T2 inoperable NSCLC (Table 12.1).
As the encouraging results of SBRT for early stage lung cancer from Timmerman et al. and the Japanese became available, the interest in SBRT has grown rapidly. RTOG 02-36 investigated 54 Gy in 3 fractions for patients with clinically staged T1 or T2a (less than 5 cm in diameter), N0M0 NSCLC, and comorbid conditions precluding surgery. Tumors within 2 cm of the proximal bronchial tree were not allowed. The 3-year primary tumor control rate was 97.6 % and the 3-year local-regional control rate was 87.2 % [13]. The use of SBRT in centrally located tumors is being further investigated in RTOG 0813.
A population-based study in the Netherlands found that as the use of SBRT became available in 2002–2004 and was widely used in 2005–2007, the percentage of patients with stage I NSCLC choosing no active treatment declined and the median survival improved for all patients [23]. Another single institution study compared patients with clinical T1 or T2N0M0 NSCLC who received wedge resection or SBRT. The overall survival was higher in those who received wedge resection, but cause-specific survival was identical and the SBRT patients had reduced local recurrence [24]. RTOG is currently evaluating the role of SBRT in the treatment of patients with operable stage I/II NSCLC in a phase II study (RTOG 06-18). In addition, two phase III randomized trials (NCT00687986 and NCT00840749) will randomize patients with early stage NSCLC to surgical resection or SBRT. As results from current trials become available the role of SBRT in NSCLC will continue to evolve.
Combined Modality Therapy
Non-small Cell Lung Cancer
Resectable NSCLC
Neoadjuvant chemoradiotherapy (CRT) is utilized in certain instances including superior sulcus tumors and selected stage IIIA (N2) disease. In Southwest Oncology Group (SWOG) trial, 9,416 patients with clinical T3–4, N0–1 superior sulcus NSCLC received two cycles of cisplatin and etoposide concurrently with radiation (45 Gy). Patients with stable or responding disease underwent thoracotomy. After completion of neoadjuvant therapy, 80 % underwent thoracotomy and 76 % had complete resection. A complete response or minimal microscopic disease was seen in 65 % of thoracotomy specimens. Five-year survival was 44 % for all patients and 54 % after complete resection [25]. This was a significant improvement over resection rates with RT and surgery of 50 %.
SWOG-8805 confirmed the feasibility of concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stage III NSCLC. Intergroup 0139 showed that induction CRT followed by surgical resection for resectable stage III NSCLC patients improved progression-free survival compared to CRT alone [26]. The lack of survival benefit (27 % vs. 20 %) was largely attributed to excess mortality related to right pneumonectomy. The subgroup of patients receiving lobectomy had significantly improved 5-year survival (36 % vs. 18 %) compared to CRT alone. It appears that induction CRT followed by surgery may benefit resectable stage IIIA patients who do not need pneumonectomy. RTOG 04-12 is further evaluating the role of neoadjuvant CRT by comparing induction chemotherapy with induction CRT followed by surgery and consolidation chemotherapy for resectable stage IIIA NSCLC.
Unresectable NSCLC
Concurrent CRT is the standard of care in locally advanced, unresectable NSCLC. RT alone is used for patients who are not candidates for chemotherapy due to poor Karnofsky performance status (KPS), old age, or medical comorbidities. Poor outcomes are obtained in such patients treated with RT alone. The role of chemotherapy in locally advanced NSCLC was established with the publication of a randomized trial (CALGB-8433) of induction chemotherapy plus high-dose radiation vs. radiation alone in stage III NSCLC by Dillman et al. [27]. In patients with stage III NSCLC, induction chemotherapy with cisplatin and vinblastine before radiation significantly improved median survival (by about 4 months) and doubled the number of long-term survivors, as compared to radiation therapy alone. A 7-year update confirmed these results [28]. RTOG 88-08 conducted a phase III trial comparing standard radiation therapy, induction chemotherapy followed by standard radiation therapy, and twice-daily radiation therapy in patients with surgically unresectable stage II, IIIA, or IIIB NSCLC. Patients were required to have a KPS of 70 or more and less than 5 % weight loss. Ninety-five percent of enrolled patients were stage III. The chemotherapy plus radiotherapy arm was statistically superior to the other two treatment arms [29]. Mature results of this trial reported that median survival for standard radiation, CRT, and hyperfractionated radiotherapy was 11.4, 13.2, and 12 months, respectively. The respective 5-year survivals were 5 % for standard RT, 8 % for chemotherapy followed by radiation therapy, and 6 % for hyperfractionated (HFX) RT [30].
RTOG conducted a phase III randomized trial (RTOG 94-10) comparing sequential and concurrent chemotherapy with daily and hyperfractionated RT. Five-year survival was 10 % for sequential chemotherapy, 16 % for concurrent daily RT, and 13 % for concurrent HFX RT [31]. A French randomized trial of sequential and concurrent reported that 2-, 3-, and 4-year survival rates were better in the concurrent arm (39 %, 25 %, and 21 %, respectively) than in the sequential arm (26 %, 19 %, and 14 %, respectively). Although the results were not statistically significant, the trend favored the concurrent CRT arm [32]. Finally, a meta-analysis of concurrent vs. sequential CRT for locally advanced NSCLC revealed a 5.7 % overall survival benefit to concurrent CRT at 5 years [33].
Concurrent CRT with daily RT is the current standard of treatment for unresectable NSCLC (Fig. 12.4). Doses of radiation range from 60 to 70 Gy with careful 3D CT guided planning with a low risk of adverse effects. RTOG 01-17 established the maximum tolerated dose of radiotherapy in the setting of concurrent chemotherapy as 74 Gy [34]. However, RTOG 06-17, which compares high (74 Gy) and low (60 Gy) radiotherapy as well as the addition of cetuximab to carboplatin and taxol, recently closed the high-dose arm due to futility. Thus, the optimum dose of radiotherapy in the setting of concurrent chemotherapy is unclear. Optimum results also depend on timely completion of RT. Machtay et al. evaluated effect of overall treatment time on outcomes after concurrent CRT for locally advanced NSCLC treated on RTOG trials. In multivariate analysis of treatment time as a continuous variable, prolonged treatment time was significantly associated with poorer survival (p = 0.02), indicating a 2 % increase in the risk of death for each day of prolongation in therapy [35].
A 3D conformal radiotherapy (3DCRT) plan for an elderly woman with a cT2aN1M0 adenocarcinoma of the left upper lobe. A 4D CT was done at the time of simulation and the tumor contoured at all phases of the breathing cycle to generate an ITV. An additional expansion was then added for setup uncertainty to generate the planning target volume (PTV). The prescription dose was 70 Gy in 35 fractions
Small Cell Lung Cancer
Limited Stage Small Cell Lung Cancer
The standard of care for limited stage small cell lung cancer (LS-SCLC) is chemoradiotherapy followed by PCI in patients with complete response to local treatment in the chest. SCLC is characterized by its propensity for early metastasis and rapid doubling time [36]. SCLC is considered a systemic disease; therefore, the role of chemotherapy is very important. Perry et al. evaluated the role of thoracic RT (TRT) in a prospective, randomized trial [37]. Patients were randomly assigned to receive initial radiotherapy pluschemotherapy, delayed radiotherapy plus chemotherapy, or chemotherapy alone. Chemotherapy was given every 3 weeks for 18 months. The radiotherapy comprised 40 Gy in 4 weeks,followed by a 10 Gy “boost” directed against residual disease. All patients received prophylactic whole brain radiation. The addition of thoracic radiotherapy to combination chemotherapy improved both complete response rates and survival, with increased but acceptable toxicity. Several meta-analyses have confirmed the benefit of RT in LS-SCLC. Pignon in a 1992 meta-analysis showed a 14 % reduction in mortality rate and 5.4 % improvement in overall survival at 3 years [38]. Warde et al. published another meta-analysis that revealed 5.4 % overall survival benefit at 2 years with the addition of TRT [39].
The optimal timing of TRT was studied in a NCI-Canada study that randomized LS-SCLC patients to early TRT (40 Gy in 15 fractions over 3 weeks to the primary site) concurrent with the first cycle of chemotherapy (week 3) and late TRT patients who received the same radiation concurrent with the last cycle of chemotherapy (week 15) [40]. Median progression-free survival was 15.4 months in the early TRT group compared to 11.8 months in the late radiation group (p = 0.036). Median overall survival was 21.2 vs. 16 months, favoring the early RT group (p = 0.008). A phase III study of concurrent vs. sequential thoracic radiotherapy in combination with cisplatin and etoposide for LS-SCLC was conducted by the Japan Clinical Oncology Group. TRT consisted of 45 Gy over 3 weeks (1.5 Gy twice daily). All patients received four cycles of cisplatin plus etoposide every 3 weeks (sequential arm) or 4 weeks (concurrent arm). TRT was begun on day 2 of the first cycle of chemotherapy in the concurrent arm and after the fourth cycle in the sequential arm. Median survival time was 19.7 months in the sequential arm vs. 27.2 months in the concurrent arm. The 2-, 3-, and 5-year survival rates for patients who received sequential radiotherapy were 35.1 %, 20.2 %, and 18.3 %, respectively, as opposed to 54.4 %, 29.8 %, and 23.7 %, respectively, for the patients who received concurrent RT [41].
The optimum dose and fractionation of TRT had not yet been determined. Due to rapid tumor repopulation, accelerated and hyperfractionated RT has been evaluated. A prospective, randomized phase III study (Intergroup 0096) compared twice-daily RT with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. Patients were assigned to receive a total of 45 Gy of concurrent TRT, given either twice daily over a 3-week period or once daily over a period of 5 weeks beginning with first cycle of chemotherapy. After a median follow-up of almost 8 years, 2- and 5-year OS was 47 % and 26 % for twice-daily arm compared to 41 % and 16 % for once-daily arm, respectively. Grade 3 esophagitis was significantly more frequent in the twice-daily group at 27 % vs. 11 % [42]. This study has been criticized because 45 Gy in 1.8 Gy daily fractions is not biologically equivalent to 45 Gy in 1.5 Gy twice-daily fractions. The duration of treatment is also shorter by 2 weeks in the BID arm. Currently, patients are either treated with twice-daily RT to 45 Gy in 1.5 Gy per fraction or to 60 Gy in 2 Gy daily fractions with concurrent chemotherapy. CALGB 30610 is currently investigating the optimal dose and fractionation in LS-SCLC and randomizes patients to 45 Gy at 1.5 Gy twice-daily fractions, 70 Gy at 2 Gy daily fractions, or 61.2 Gy at 1.8 fractions daily with a concomitant boost of 1.8 Gy for the last 9 treatment days.
Extensive Stage SCLC
Chemotherapy alone is the mainstay of treatment in extensive stage SCLC (ES-SCLC) and RT is utilized either for PCI in good responders [43] or for palliative treatment in bone or brain metastasis or for relief of airway or superior vena cava obstruction. The role of consolidative TRT in ES-SCLC was examined in patients with a CR or PR in the thorax and a CR at distant sites after three cycles of cisplatin and etoposide. Patients were randomized to four additional cycles of cisplatin and etoposide or 54 Gy in 36 fractions TRT over 18 treatment days with carboplatin and etoposide followed by two cycles of cisplatin and etoposide. All patients received PCI. Five-year survival was improved in the TRT arm at 9.1 % vs. 3.7 % in the chemotherapy alone arm [44]. However, thoracic RT is not widely accepted in ES-SCLC. RTOG 09-37 is a phase II trial that further examines the role of RT in ES-SCLC by randomizing patients with up to 1–4 sites of extracranial metastatic disease and partial or complete response after 4–6 cycles of platinum-based chemotherapy to PCI alone or PCI and consolidative extracranial irradiation.
Adjuvant Radiotherapy in NSCLC
The use of adjuvant RT in completely resected NSCLC remains controversial. Lung Cancer Study Group (LCSG) 773 assessed the effect of mediastinal RT (50 Gy in 5–5.5 weeks) following resection of stage II and III squamous cell carcinoma of lung. A marked reduction in local relapse as first site of failure from 41 to 3 % was observed for patients receiving postoperative RT (PORT), but this was without survival benefit. A subgroup analysis of N2 patients revealed a trend towards a survival benefit.
A meta-analysis of PORT [45] showed that there was a 21 % relative increase in the risk of death in patients treated with PORT, equivalent to an absolute detriment of 7 % at 2 years, reducing overall survival from 55 to 48 %. Subgroup analyses suggested that this adverse effect was greatest for patients with stage I/II, N0–N1 disease. For those with stage III, N2 disease, there was no clear evidence of an adverse effect. This detriment was presumably due to increase in intercurrent deaths. Results of this analysis were widely criticized for many reasons including methodological issues and including series with outdated RT modality, technique, and inappropriate radiation dose and fractionation [46]. A Mayo Clinic retrospective review to determine the local recurrence and survival rates for patients with N2 disease undergoing complete surgical resection with or without PORT revealed that actuarial 4-year local recurrence rate was 60 %, compared with 17 % for PORT (p < 0.0001) [47]. The actuarial 4-year survival rate was 22 % for treatment with surgery alone, compared with 43 % for treatment with PORT. Recent studies using modern RT have failed to show a detrimental effect of PORT [48]. A phase III trial of adjuvant radiotherapy in NSCLC with pathological stage I randomized pathologic staged IA and IB patients to PORT or observation. Local recurrence rate was 2.2 % in the RT group compared to 23 % in the observation group. Overall 5-year survival (Kaplan–Meier) showed a positive trend in the treated group: 67 % vs. 58 %. Treatment-related toxicity was acceptable [49].
Recently, the Adjuvant Navelbine International Trialist Association (ANITA) randomized trial analyzed the results of patients receiving PORT in the phase III randomized trial of adjuvant chemotherapy following surgery. Approximately 30 % of patients received PORT. They concluded that PORT had a beneficial effect in the pathologic N2 subgroup of patients [50]. The European Organization for the Research and Treatment of Cancer (EORTC) is launching a large randomized trial to further evaluate the role of adjuvant RT in N2 disease. Adjuvant RT is advisable for positive/close margins of resection in T1–T2 N0–1 disease. Mediastinal RT to 50 Gy in 1.8–2 Gy per fraction using 3D conformal RT may be considered for selected pathologic N2 patients, particularly with multi-station nodal involvement (Fig. 12.5). An excellent review by Bogart et al. can provide further insights [46].
Palliative Radiotherapy
Radiation therapy is very effective in palliating symptoms of metastatic lung cancer [51]. Symptoms of pain from bone metastasis, chest wall invasion by primary tumor, and radicular pain from nerve root invasion can be palliated by RT. Prompt radiotherapy for spinal cord compression, superior vena cava syndrome, and brain metastasis can help relieve symptoms or preempt development of more serious effects. Brain metastases are usually treated with surgery, whole brain RT (WBRT), and/or stereotactic radiosurgery (SRS). Optimal treatment of brain metastases depends on age, primary site, control of the primary, interval to development of brain metastases, disease-free interval, number of brain metastases, presence of extracranial metastases, KPS, treatment of brain metastases, and recursive partitioning analysis (RPA) class. A phase III randomized trial of WBRT with or without SRS boost for patients with 1–3 brain metastases (RTOG 95-08) concluded that WBRT and stereotactic boost treatment improved functional autonomy (KPS) for all patients and survival for patients with a single unresectable brain metastasis [52]. In a randomized trial of surgery followed by WBRT compared to WBRT alone in the treatment of single metastases to the brain, Patchell et al. concluded that patients with a single metastasis to the brain who receive treatment with surgical resection plus radiotherapy live longer, have fewer recurrences of cancer in the brain, and have a better quality of life than similar patients treated with radiotherapy alone [53]. Another randomized trial by the same group compared postoperative WBRT with surgery alone. Patients with single metastases to the brain who received treatment with surgical resection and postoperative radiotherapy had fewer recurrences of cancer in the brain and were less likely to die of neurologic causes than similar patients treated with surgical resection alone [54]. The RTOG is currently conducting a phase III randomized, double blind, placebo-controlled trial of Memantine for prevention of cognitive dysfunction in patients receiving WBRT (RTOG 06-14).
Other examples of palliation include relief of airway obstruction to improve symptoms of dyspnea or prevention of recurrent post-obstructive pneumonia. RT is also very effective in treating hemoptysis arising from ulcerative lesions in the lung. Sometimes, endobronchial bleeding lesions are treated with intraluminal brachytherapy in single or multiple fractions. The usual radiotherapy dose for palliation ranges from 37.5 Gy in 15 fractions to 20 Gy in 5 fractions given daily, 5 days a week. The type and duration of treatment depends on the KPS of the patient and type and status of the cancer.
A randomized trial of single treatment with 8 Gy compared to 30 Gy in 10 fractions was conducted in patients with painful bone metastases [55]. Both regimens were equivalent in terms of pain and narcotic relief at 3 months and were well tolerated with few adverse effects. A meta-analysis of fractionated radiotherapy trials for the palliation of painful bone metastases showed no significant difference in complete and overall pain relief between single and multi-fraction palliative RT for bone metastases [56].
Prophylactic Cranial Irradiation
The brain is an important site of disease failure in lung cancer and is associated with poor prognosis. Overall CNS failure after potentially curative local therapy ranges from 21 to 54 % [57, 58]. Brain metastases are common in adenocarcinoma, large cell carcinoma, and locally advanced disease. Stuschke et al. treated patients with stage III NSCLC with PCI and reduced the rate of brain metastases as first site of relapse from 30 to 8 % at 4 years (p = 0.005) and overall brain relapse from 54 to 13 % (p < 0.0001) [57]. The late toxicity to normal brain was acceptable. RTOG conducted a phase III randomized trial (RTOG 02-14) of PCI compared to observation in patients with stage III NSCLC with stable extracranial disease 4 months after completion of their initial treatment. The study was closed early due to slow accrual. There was no difference in overall survival or disease-free survival at 1 year, but rates of brain metastasis at 1 year were significantly lower in the PCI arm (7.7 % vs. 18 %) [59]. Currently, PCI is not recommended for patients with NSCLC outside of a clinical trial.
Brain metastases are common in SCLC. Arriagada et al. conducted a prospective study of PCI compared to no PCI in 300 patients with SCLC in complete remission. The 2-year cumulative rate of brain metastasis as an isolated first site of relapse was 45 % in the control group and 19 % in the treatment group (p < 10(−6)). The total 2-year rate of brain metastasis was 67 % and 40 %, respectively (relative risk = 0.35; p < 10(−13)). The 2-year overall survival rate was 21.5 % in the control group and 29 % in the treatment group (relative risk = 0.83; p = 0.14). There were no significant differences between the two groups in terms of neuropsychological function or abnormalities indicated by brain CT scans [60]. A meta-analysis of seven randomized trials of PCI in SCLC showed that PCI improved both overall survival (5.4 % at 3 years) and disease-free survival among patients with SCLC in complete remission [61]. RTOG 02-12 compared 25 Gy in 10 fractions to 36 Gy (18 daily fractions or 24 twice-daily fractions) of PCI in patients with limited stage SCLC in complete remission. The 2-year incidence of brain metastases and overall survival were the same in both arms and 25 Gy is considered the standard for PCI in limited stage SCLC [62].
Slotman and colleagues recently published results of a phase III randomized trial of PCI in ES-SCLC patients who had a response to chemotherapy [43]. The primary end point was the time to symptomatic brain metastases. Several dose and fractionation schedules were allowed and the one most commonly used was 20 Gy in 5 fractions. The cumulative risk of brain metastases within 1 year was 14.6 % in the irradiation group and 40.4 % in the control group. Irradiation was associated with an increase in median disease-free survival from 12.0 to 14.7 weeks and in median overall survival from 5.4 to 6.7 months after randomization (Fig. 12.6). The 1-year survival rate was 27.1 % in the irradiation group and 13.3 % in the control group.
Toxicity of Radiation Therapy
Radiation therapy delivered either alone or in combination with chemotherapy or surgery may cause both acute and late side effects due to the effect of RT on normal tissue and organ systems. Recent advances in imaging, technique, and delivery of RT such as IGRT and IMRT have improved target accuracy and normal tissue avoidance, but increased heterogeneity can lead to increased “hotspots” in surrounding tissues. Most common acute side effects of thoracic RT (during and up to 3 months following treatment) include fatigue, radiation dermatitis (usually in RT portals), dyspnea, and esophagitis. Late effects include radiation pneumonitis (RP), esophageal stricture/perforation, risk of rib fracture, cardiac toxicity (including pericardial disease, valvular disease, and myocardial infarction), spinal cord injury, and brachial plexopathy.
Esophagitis leads to pain in swallowing and poor oral intake leading to dehydration, electrolyte imbalance, and weight loss. Approximately 50–70 % patients receiving concomitant chemoradiotherapy develop grade 1 or 2 esophageal toxicity. A retrospective study of predictors of radiation-induced esophageal toxicity in patients with NSCLC treated with three-dimensional conformal radiotherapy showed that concurrent chemotherapy and the maximal esophageal point dose (58 Gy) were significantly associated with a risk of grade 3–5 esophageal toxicity [63]. Esophagitis is treated with diet modifications, mucositis rinses, antacids, and pain medications. Esophageal strictures are managed by dilatations.
Radiation pneumonitis (RP) is inflammation of the lungs as a result of radiation. It usually manifests itself 2 weeks to 6 months after completion of RT. Symptoms include dyspnea on exertion, cough, and low grade fever. RP is an interstitial pulmonary inflammation that can develop in as many as 5–15 % of patients with thoracic irradiation (Fig. 12.7). Classical RP involves direct toxic injury to endothelial and epithelial cells from the radiation, resulting initially in an acute alveolitis. This process leads to an accumulation of inflammatory and immune effector cells within the alveolar walls and spaces. The accumulation of leukocytes distorts the normal alveolar structures and results in the release of lymphokines and monokines. The alveolar macrophage is thought to play a central role in the subsequent development of chronic inflammation. Sporadic RP results in an “out-of-field” response. This is thought to be an immunologically mediated process resulting in bilateral lymphocytic alveolitis. The severity of RP may range from asymptomatic X-ray changes to severe respiratory compromise requiring ventilator support. Some of the factors determining risk and degree of RP include prior lung disease, history of lung resection, volume of lung treated and dose (mean lung dose [MLD]), chemotherapy, and performance status.
Several investigators have published data regarding predictors of RP. Bradley and colleagues reviewed data from RTOG 9311 and their institutional dataset and concluded that there was greater risk of RP due to inferior lung irradiation and increasing normal lung mean dose [64]. A prospective study showed that MLD, V20, and V30 (volume of lung receiving 20 Gy and 30 Gy, respectively) were associated with severe RP [65]. Another investigator concluded V10 and V13 as the best predictors of RP risk, with a decrease in predictive value above those volumes [66]. Borst et al. looked at pulmonary function changes after radiotherapy in NSCLC patients with long-term disease-free survival and suggested that a significant decrease in pulmonary function was observed 3 months after RT. No recovery in pulmonary function was seen at 18 and 36 months after RT. The decrease in pulmonary function was dependent on the MLD, and patients with chronic obstructive pulmonary disease had larger reductions in the PFTs [67]. Most recently, the QUANTEC (quantitative analyses of normal tissue effects in the clinic) group recommended maintaining V20 ≤ 30–35 % and MLD ≤ 20–23 Gy to limit the risk of RP to ≤20 % [68]. Treatment of RP is steroids, oxygen, antibiotics for infection, and ventilator support if needed.
References
Tyldesley S, Boyd C, Schulze K, Walker H, Mackilop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49(4):973–85.
Kong FM, Zhao L, Hayman JA. The role of radiation therapy in thoracic tumors. Hematol Oncol Clin North Am. 2006;20(2):363–400.
Decker RH, Tanoue LT, Colasanto JM, Detterbeck FC, Wilson LD. Evaluation and definitive management of medically inoperable early-stage non-small-cell lung cancer. Part 1: assessment and conventional radiotherapy. Oncology (Williston Park). 2006;20(7):727–36.
Zimmermann FB, Bamberg M, Molls M, Jeremic B. Radiation therapy alone in early stage non-small cell lung cancer. Semin Surg Oncol. 2003;21:91–7.
Narayan S, Henning GT, Ten Haken RK, Sullivan MA, Martel MK, Hayman JA. Results following treatment to doses of 92.4 or 102.9 Gy on a phase I dose escalation study for non-small cell lung cancer. Lung Cancer. 2004;44(1):79–88.
Urbanic JJ, Turrisi AT, Sharma AK, et al. Conformal high dose external radiation therapy, 80.5 Gy, alone for medically inoperable non-small cell lung cancer: a retrospective analysis. J Thorac Oncol. 2006;1(2):112–9.
Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–28.
Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3(2):177–86.
Heinzerling JH, Anderson JF, Papiez L, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys. 2008;70(5):1571–8.
Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124(5):1946–55.
Timmerman R, McGarry R, Tiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–9.
Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100.
Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6.
Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45(7):787–95.
Fritz P, Kraus HJ, Muhlnickel W, et al. Stereotactic, single-dose irradiation of stage I non-small cell lung cancer and lung metastases. Radiat Oncol. 2006;1:30.
Nyman J, Johansson KA, Hulten U, et al. Stereotactic hypofractionated radiotherapy for stage I non-small cell lung cancer—mature results for medically inoperable patients. Lung Cancer. 2006;51(1):97–103.
Zimmermann FB, Geinitz H, Schill S, et al. Stereotactoc hypofractionated radiation therapy for stage I non-small cell lung cancer. Lung Cancer. 2005;48(1):107–14.
Matsuo Y, Shibuya K, Nagata Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79(4):1104–11.
Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiotherapy for patients with inoperable stage I/II non-small cell-lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(1):117–25.
Hara R, Itami J, Aruga T, et al. Clinical outcomes of single-fraction stereotactic radiation therapy of lung tumors. Cancer. 2006;106(6):1347–52.
Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risk in small-volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56(1):126–35.
Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63(5):1427–31.
Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28(35):5153–9.
Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928–35.
Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol. 2007;25(3):313–8.
Turrisi AT, Scott C, Rusch V, et al. Randomized trial of chemoradiotherapy to 61 Gy [no S] versus chemoradiotherapy to 45 Gy followed by surgery [S] using cisplatin etoposide in stage IIIa non-small cell lung cancer (NSCLC): intergroup trial 0139, RTOG (9309). Int J Radiat Oncol Biol Phys. 2003;57:S125–6.
Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323:940–5.
Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst. 1996;88:1210–5.
Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88–08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst. 1995;87:198–205.
Sause W, Kolesar P, Taylor S, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000;117:358–64.
Curran W, Paulus R, Langer C, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1–9.
Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95–01 Study. J Clin Oncol. 2005;23(25):5910–7.
Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90.
Bradley J, Moughan J, Graham MV, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys. 2010;77:367–72.
Machtay M, Hsu C, Komaki R, et al. Effect of overall treatment time on outcomes after concurrent chemoradiation for locally advanced non-small-cell lung carcinoma: analysis of the Radiation Therapy Oncology Group (RTOG) experience. Int J Radiat Oncol Biol Phys. 2005;63:667–71.
Simon G, Ginsberg RJ, Ruckdeschel JC. Small-cell lung cancer. Chest Surg Clin N Am. 2001;11(1):165–88; ix.
Perry MC, Eaton WL, Propert KJ, et al. Chemotherapy with or without radiation therapy in limited small-cell carcinoma of the lung. N Engl J Med. 1987;316(15):912–8.
Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327(23):1618–24.
Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10(6):890–5.
Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11(2):336–44.
Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20(14):3054–60.
Turrisi III AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340(4):265–71.
Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–72.
Jeremic B, Shibamoto Y, Nikolic N, et al. Role of radiation therapy in the combined-modality treatment of patients with extensive disease small-cell lung cancer: a randomized study. J Clin Oncol. 1999;17:2092–9.
Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. PORT Meta-analysis Trialists Group. Lancet. 1998;352:257–63.
Bogart JA, Aronowitz JN. Localized non-small cell lung cancer: adjuvant radiotherapy in the era of effective systemic therapy. Clin Cancer Res. 2005;11(13 Pt 2):5004s–10s.
Sawyer TE, Bonner JA, Gould PM, et al. The impact of surgical adjuvant thoracic radiation therapy for patients with nonsmall cell lung carcinoma with ipsilateral mediastinal lymph node involvement. Cancer. 1997;80:1399–408.
Machtay M, Lee JH, Shrager JB, Kaiser LR, Glatstein E. Risk of death from intercurrent disease is not excessively increased by modern postoperative radiotherapy for high-risk resected non-small-cell lung carcinoma. J Clin Oncol. 2001;9(19):3912–7.
Trodella L, Granone P, Valente S, et al. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: definitive results of a phase III randomized trial. Radiother Oncol. 2002;62(1):11–9.
Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72(3):695–701.
Konski AS, Feigenberg S, Chow E. Palliative radiation therapy. Semin Oncol. 2005;32(2):156–64.
Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–72.
Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494–500.
Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485–9.
Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798–804.
Wu JS, Wong R, Johnston M, Bezjak A, Whelan T, Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55(3):594–605.
Stuschke M, Eberhardt W, Pottgen C, et al. Prophylactic cranial irradiation in locally advanced non-small-cell lung cancer after multimodality treatment: long-term follow-up and investigations of late neuropsychologic effects. J Clin Oncol. 1999;17(9):2700–9.
Andre F, Grunenwald D, Pujol JL, et al. Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy: should prophylactic cranial irradiation be reconsidered? Cancer. 2001;91(12):2394–400.
Gore EM, Bae K, Wong SJ, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol. 2010;29(3):272–8.
Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87(3):183–90.
Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341(7):476–84.
Le Péchoux C, Dunant A, Senan S, et al. Stand-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003–08004, RTOG 0212, and IFCT 99–01): a randomised clinical trial. Lancet Oncol. 2009;10(5):467–74.
Singh AK, Lockett MA, Bradley JD. Predictors of radiation-induced esophageal toxicity in patients with non-small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55(2):337–41.
Bradley JD, Hope A, El Naga I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69(4):985–92.
Claude L, Pérol D, Ginestet C, et al. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol. 2004;71(2):175–81.
Schallenkamp JM, Miller RC, Brinkmann DH, et al. Incidence of radiation pneumonitis after thoracic irradiation: dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67(2):410–6.
Borst GR, De Jaeger K, Belderbos JS, Burgers SA, Lebesque JV. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys. 2005;62(3):639–44.
Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cooper, S.L., Sharma, A. (2013). Radiotherapy in Lung Cancer. In: Ravenel, J. (eds) Lung Cancer Imaging. Contemporary Medical Imaging. Humana Press, New York, NY. https://doi.org/10.1007/978-1-60761-620-7_12
Download citation
DOI: https://doi.org/10.1007/978-1-60761-620-7_12
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-60761-619-1
Online ISBN: 978-1-60761-620-7
eBook Packages: MedicineMedicine (R0)