Abstract
This study examined the dynamic impact of self-control conflict on action execution. We reasoned that the tug-of-war between antagonistic action tendencies is not ultimately solved before movement initiation but leaks into action execution. To this end, we measured mouse trajectories to quantify the dynamic competition between initial temptations and the struggle to overcome them. Participants moved the mouse cursor from a start location to one of two targets. Each target represented a gain or a loss of points. Although participants earned points on the majority of the trials, they also had to make movements to the loss target on some trials to prevent an even higher loss. Two experiments found that movement trajectories on these loss trials deviate toward the tempting stimulus: The way we move reveals self-control conflicts that have not been resolved prior to action execution.
Similar content being viewed by others
In everyday life, we often have to resist momentary temptations and pleasures for a greater good (Metcalfe & Mischel, 1999; Muraven & Baumeister, 2000). Imagine a child who has a sweet tooth and a bellyache from overeating candies. The child knows that consuming more sweets will likely exacerbate the ache, whereas consumption of a bitter stomach medicine will provide some relief. To get rid of the bellyache, the child must resist the momentary pleasure of a candy and endure the momentary displeasure of the bitter medicine. Overcoming these impulses is often a very difficult task that involves self-control. Perhaps the child will grab the medicine bottle very hesitantly, being repulsed by the bitter taste of the medicine and/or being attracted by the sweet pleasure of a candy. Furthermore, even with a very firm determination to swallow the bitter medicine, the child may exhibit a prolonged and circuitous behavior that reflects the underlying motivational conflict.
Such a self-control dilemma typically represents an internal conflict between two antagonistic behavioral tendencies: (1) a motivational impulse to maximize pleasure and/or to minimize displeasure and (2) a planned behavior that is controlled by intentions (Strack & Deutsch, 2004; Trope & Fishbach, 2000). At least, older humans have a capacity at command to deal with a self-control dilemma and to resist momentary impulses for a greater good. This capacity is called self-control (Muraven & Baumeister, 2000; for an overview, see Hofmann, Friese, & Strack, 2009). A central feature of self-control is the ability to override automatic impulses by replacing the impulsive action with a behavioral plan that is of greater importance than the impulsive response (Baumeister, 2002).
While self-control is a reasonable concept to explain how conflict is resolved before an action is initiated, it is less clear how self-control expresses itself in behavior. Most research on self-control has examined outcomes of decision processes, which fits with the assumption of traditional stage models of information processing that a tug-of-war between two competing action plans is resolved prior to action execution (Sternberg, 1969). The execution of the behavior itself is assumed to run more or less encapsulated from the self-control operations, which explains why movement execution has rarely been investigated in self-control research.
An alternative approach has been advanced by continuous information processing models (Miller, 1988). According to these models, the stream of information from perception to action is continuous and not divided into separate processing stages (Erlhagen & Schöner, 2002). In line with this approach, Spivey, Grosjean, and Knoblich (2005) showed that the competition between two choice alternatives is apparent in the trajectory of the movement after a response decision. They asked participants to move a computer mouse to a target picture, while a distractor picture was presented on the other side of the screen that could be either phonologically related to the target or not. Importantly, the trajectories of the computer mouse were recorded, which provided a detailed profile of the movement trajectories during action execution. When the names of the target and the distractor were phonologically similar, movement trajectories were attracted toward the distractor (and more so than for phonologically unrelated distractors), revealing dynamic influences of the decision process on action execution (for additional evidence, see Freeman & Ambady, 2011; Freeman, Ambady, Rule, & Johnson, 2008).
Experiment 1
The aim of the present study was to explore the dynamics of self-control in action execution. We reasoned that the tug-of-war between antagonistic response options is not ultimately solved before action initiation but leaks into action execution. To quantify a dynamic competition between planned goal-directed actions and temptations, we measured movement trajectories of the computer mouse. Participants moved a virtual manikin on the computer screen to one of two target areas—a win and a loss target. Approaching the win target was rewarded with 5 points; in contrast, 5 points were subtracted from the total score when the loss target was approached. On each trial, a movement cue indicated whether the manikin should be moved to the win or to the loss target. To ensure a rewarding task structure, the manikin was moved to the win target more frequently than to the loss target. Performing an erroneous response to the loss target on win trials resulted in a subtraction of 5 points, while performing an erroneous response to the win target on loss trials was punished with a subtraction of 10 points. Thus, correctly approaching the loss target on loss trials (i.e., the bitter medicine of the introductory example) subtracted 5 points, while approaching the incorrect win target (i.e., sweets) produced an even greater loss (10 points) on these trials.
We reasoned that on loss trials, movement execution is influenced by behavioral impulses to avoid the (correct) loss target and/or to approach the (incorrect) win target. This internal conflict between motivational impulses, on the one hand, and an intended action, on the other hand, reflects a self-control dilemma. Thus, although our participants agreed to comply with the task instructions, movement execution might reveal a self-regulatory conflict. We expected that movement trajectories on the loss trials would deviate from trajectories on the win trials. Specifically, we hypothesized that trajectories would deviate away from the loss target toward the tempting win target on the loss trials, even if the manikin finally reached the loss target.
As was mentioned above, win trials were more frequent than loss trials, to maintain a rewarding task structure. Losing too many points likely causes frustration, and participants may not pay attention to the wins and losses if nothing can be gained. Second, from a theoretical point of view, self-control can be studied only if the task requires participants to overcome impulses triggered by temptations. To ensure that a stimulus acts as a temptation, either the stimulus has to already possess an inherent positive valence (e.g., food), or the stimulus has to acquire a positive valence by task procedures. Getting more money than losing money after the execution of a movement arguably endows the corresponding movement target with a positive valence (Schultz, 2006). In addition, more frequent exposure to the win target makes it more likely that the win target will acquire this positive valence. Consequently, participants are attracted by the win-related target, and they have to overcome a spontaneous impulse to move toward the win target (and/or to avoid the loss-related target) when they are instructed to perform a movement toward the loss-related target on loss trials. However, given that the majority of the trials involved a movement to the win target, any differences in the movement trajectories could be due to the unbalanced target frequencies. We addressed this obvious confound with a control condition. The control condition was essentially the same as the conflict condition, but without involving a motivational conflict. Thus, we expected smaller or no differences between win and loss trials in the movement trajectories for the control condition (without a motivational conflict), relative to the conflict condition (with a motivational conflict). Because win and loss trials implied wins and losses only in the conflict condition, we will refer to the two trial types as high-frequent and low-frequent trials for the control condition.
Method
Participants
Seventeen participants (14 women, 1 left-handed) were recruited for the conflict condition, and 18 participants (16 women, 2 left-handed) were recruited for the control condition. Participants were between 18 and 25 years of age and received monetary compensation. One participant in the conflict condition had to be excluded due to unusual high error rates (12.6 %) on both win and loss trials (>2.5 SDs).
Apparatus and stimuli
Participants performed their responses with a standard computer mouse (“Fujitsu Notebook Mouse 400NB,” Fujitsu Technology Solutions GmbH, Germany), and mouse trajectories were sampled with a frequency of 200 Hz. Stimuli were presented on a 17-in. screen running at a resolution of 1,024 × 768 pixels. Three empty boxes (50 × 50 pixels) were displayed on a black background. Two target boxes designating target areas were presented in the upper third of the screen, one to the left (midpoint in pixels: x = 281, y = 217) and one to right (x = 793, y= 217; coordinates are given relative to the upper left corner of the screen). Each target box contained a picture of either a smiley or a frowny (see Fig. 1). Location of smiley and frowny pictures changed randomly from trial to trial. An empty start box was displayed in the lower center of the screen (x = 512, y = 709). The mouse cursor displayed a small manikin (28 × 50 pixels). In the conflict condition, we used an ascending tone (win trials) and a descending tone (loss trials) with a duration of 400 ms to indicate that participants had reached the target area. Ascending and descending tones were chosen to make the mapping of wins and losses to the target location more obvious for the participants. In the control condition, a neutral 400 or 600 Hz tone was presented for 400 ms.
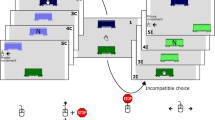
Upper panels: Trial sequence for Experiment 1. Participants moved the manikin with the computer mouse into the start area at the bottom of the screen. Subsequently, a cue specified the correct response, and auditory and visual feedback was provided when participants arrived at one of the target boxes. Lower panels: Mean movement trajectory for the two conditions (loss-trials/low-frequency trials vs. win-trials/high-frequency trials) for the conflict condition (left side) and the control condition (right side) in Experiment 1
Procedure and design
At the start of a trial, a movement cue (+ or −) appeared in the start box (see Freeman & Ambady, 2009, for a similar procedure). If a “+” appeared in the box, participants were to move the manikin as quickly as possible to the smiley box, resulting in a win of 5 points. If the cue was a “−,” participants were to move the manikin to the frowny box, resulting in a loss of 5 points. Ten points were subtracted when the manikin did not reach the designated target within 2 s. A counter in the upper left corner of the screen displayed the total point score throughout the experiment. Participants were informed that they would get a chocolate bar as a bonus reward when their score exceeded a certain (but not further defined) threshold at the end of the session.
After 10 training trials on which no points were earned or lost, participants worked through 200 experimental trials. In the conflict condition, these trials consisted of 170 win trials and 30 loss trials. At the start of each trial, the empty start box and the target boxes with a smiley or frowny appeared on the screen. Participants initiated a trial by moving the manikin from the middle of the screen to the start box. Then the movement cue appeared in the start box after a random interval (200–400 ms). Upon arrival in one of the target areas, a tone was played, and the amount of points that were earned or lost on this trial popped up above the box (e.g., +5). If participants selected the incorrect target area or did not reach the target area within 2 s, an error message appeared on the screen for 2 s, indicating the loss of 10 points.
In the control condition, the target boxes were shown with a blue or yellow frame. One color (counterbalanced across participants) was cued on 170 of the trials (high-frequency trials), whereas the other color was cued only on 30 trials (low-frequency trials). Movement cues were the letters “b” (blue) and “g” (yellow) that appeared in the start box. Tones were a 400- or 600-Hz tone without affective connotation. Importantly, participants could not earn or lose points in this condition. In case of a correct response, “ok” popped up above the box. In case of a time-out or a wrong response, an error message was displayed on the screen for 2 s.
Data processing
Reaction time (RT) was measured from onset of the cue until the manikin left the start box, and movement time (MT) was measured as the time between leaving the start box and arriving at the target box. Trials with RTs or MTs deviating more than 2.5 standard deviations from the participant’s mean RT or MT were discarded, calculated separately for win/high-frequency trials and loss/low-frequency trials (conflict condition, 4.6 %; control condition, 4.8 %). Trials with time-outs (conflict condition, <.01 %; control condition, no omissions), response anticipations (i.e., when the manikin left the start box before cue onset; conflict condition, 7.3 %; control condition, 7.5 %) and incorrect responses (conflict condition, 2.1 %; control condition, 0.7 %) were excluded from the analyses. Each trajectory was aligned to a common starting position (horizontal middle position) and time-normalized to 101 equidistant time slices, excluding the data of the RT interval. Trajectories to the left side were mirrored for analysis. We then calculated two additional dependent variables for each trial: maximum absolute distance (MAD) and area under the curve (AUC). MAD is defined as the maximum distance between a trajectory and a straight line from the start point to the end point of this trajectory. MAD is coded positive for curves toward the alternative target and negative for curves away from it. AUC is defined as the area between the trajectory and the straight line. Trials with MADs or AUCs deviating more than 2.5 standard deviations from the participant’s mean MAD or AUC were discarded, calculated separately for win/high-frequency trials and loss/low-frequency trials (conflict condition, 3.9 %; control condition, 3.9 %).
Results
For a direct comparison of the conflict and the control conditions, we first submitted the data to mixed ANOVAs with condition (conflict vs. control) as a between-subjects factor and trial type (conflict condition, win trials vs. loss trials; control condition, high-frequency vs. low-frequency trials) as a within-subjects factor. For all dependent variables, the interaction of condition and trial type was significant or approached significance, indicating that the impact of trial type differed between conditions [RT: F(1, 32) = 16.01, p < .001, η p 2 = .33; MT, F(1, 32) = 4.49, p < .05, η p 2 = .12; error rates, F(1, 32) = 6.91, p < .05, η p 2 = .17; MAD, F(1, 32) = 6.11, p < .05, η p 2 = .16; AUC, F(1, 32) = 3.35, p = .078, η p 2 = .09]. Consequently, we analyzed the influence of trial type separately for each condition.
Conflict condition
RTs were longer on loss trials (643 ms) than on win trials (559 ms), t(15) = 6.18, p < .001, d = 1.54, and an analogous effect was observed for MT: Participants needed more time to reach the target area on loss trials (307 ms) than on win trials (270 ms), t(15) = 2.76, p < .05, d = 0.69. A comparison of the trajectory data between the two trial types revealed that movements on loss trials deviated away from the loss target toward the win target, whereas no such bias was evident on win trials (Fig. 1). Accordingly, there was a significant difference between trial types for both MAD, t(15) = 3.63, p < .005, d = 0.91, and AUC, t(15) = 3.26, p < .01, d = 0.81.
The pattern of RTs and mouse trajectories is mirrored by the error data. Errors were more frequent on loss trials (M = 5.3 %) than on win trials (M = 1.8 %), t(15) = 2.48, p < .05, d = 0.62, despite the higher penalty that incurred for loss trials.
Control condition
RTs were longer for low-frequency trials (473 ms) than for high-frequency trials (446 ms), t(17) = 4.69, p < .001, d = 1.11. However, t-tests for MT, MAD, AUC, and error rate showed no differences, all ps > .250 (Fig. 2).
Mean reaction times (RTs), movement times (MTs), maximum absolute distances (MADs), and areas under the curve (AUCs) for the two conditions (win-trials/high-frequency trials vs. loss-trials/low-frequency trials) for the conflict and the control conditions in Experiment 1. Error bars show standard errors of paired differences, computed separately for each condition (see Pfister & Janczyk, 2013, for details)
Discussion
Consistent with our predictions, movement trajectories differed markedly between loss and win trials in the conflict condition. When participants moved the manikin toward the loss target, movement trajectories were deflected more than when they moved the manikin to the win target. Conceivably, this effect in the movement trajectories is in line with the idea of an ongoing conflict between motivational impulses and instructed action intentions. A self-control conflict is also supported by increased RTs, MTs, and error rates on the loss trials. Importantly, in the control condition with the same movement frequencies but without affective-motivational consequences, only the RTs differed between high-frequency trials and low-frequency trials. Movement trajectories, however, were not different in this condition. The absence of a comparable effect in the control condition strongly argues against the possibility that the deviation of the trajectory in the conflict condition was merely driven by the infrequency of the response.
For Experiment 1, it is unclear, however, whether the effect in the movement trajectories occurred because of a biased processing of the emotional stimuli that were presented in the target area (i.e., a smiley and grumpy) or because of the associations with wins and losses. Thus, it is possible that the effect in the movement trajectories was induced by the emotional faces and not by a motivational conflict. This question is examined in Experiment 2.
Experiment 2
To provide a more conclusive test of a self-control conflict during movement execution, we conducted an additional experiment that did not present emotional stimuli. The experimental design was identical with the conflict condition of Experiment 1, with the major change that neutral colors were used to signal the target areas producing wins and losses. Thus, no emotional faces were presented in this experiment, ruling out an explanation with biased processing of emotional faces.
Method
Participants and procedure
Eighteen participants (14 women, 1 left-handed; 19–30 years) were paid for their participation.
Experiment 2 involved three major changes in comparison with the conflict condition of Experiment 1. First, win and loss targets were represented by blue or yellow target boxes (counterbalanced across participants) that replaced the emotional faces. Second, movement cues were the letters “b” and “g.” Participants were to move the manikin to the blue box when the letter “b” (German “blau,” blue) appeared in the start box and to the yellow box when the letter “g” appeared in the start box (German “gelb,” yellow). Third, no tones were presented after reaching the target box.
Participants worked through 200 experimental trials that consisted of 170 win trials and 30 loss trials. Participants were informed about the color association with wins and losses. Instructions emphasized that 5 points would be added to the point score when the manikin reached the colored box associated with a win; in contrast, 5 points would be subtracted from the total score when the manikin reached the colored box associated with a loss. Erroneous and omitted responses were penalized with a subtraction of 10 points. All other procedural details were identical to those of the conflict condition in Experiment 1.
Data processing
Trials with RTs/MTs/MADs/AUCs deviating more than 2.5 standard deviations from the participant’s mean RT/MT/MAD/AUC were discarded, calculated separately for win and loss trials (RT/MT, 4.7 %; MAD/AUC, 3.3 %). Trials with time-outs (0.03 %), response anticipations (7.7 %), and incorrect responses (0.05 %) were excluded from the analyses. Informal visual inspection of the movement data indicated that one participant showed a particularly pronounced curved trajectory. Z-transformation of the means of the trajectory data revealed that MAD and AUC of this participant exceeded 3 standard deviations from the sample’s mean MAD or AUC. Therefore, we removed this data set from the analyses. Including the participant in the analyses did not change the pattern of results.
Results
RTs were longer on loss trials (484 ms) than on win trials (430 ms), t(16) = 6.23, p < .001, d = 1.55. An analogous effect was observed for MTs: Participants needed more time to reach the target area on loss trials (336 ms) than on win trials (317 ms), t(16) = 2.35, p < .01, d = 0.74. A comparison of the trajectory data between the two trial types showed that movements on the loss trials deviated away from the loss target toward the win target and that this effect was more pronounced than the reverse deviation on win trials. This difference was significant for both MAD, t(16) = 3.77, p < .001, d = 0.88, and AUC, t(16) = 3.43, p < .001, d = 0.78.
The pattern of movement errors is in line with the RT and mouse trajectory analyses, with more errors on loss trials (M = 1.7 %) than on win trials (M = 0.3 %); this difference reached significance in a one-tailed test, t(16) = 1.78, p < .05, d = 0.43. However, this analysis has to be treated with caution, since errors were, in general, quite rare (Fig. 3).
Comparison of Experiments 1 and 2
To compare the results of Experiment 1 and Experiment 2, we submitted the data to a 3 (Experiment 1, conflict condition; Experiment 1, control condition; Experiment 2, conflict condition) × 2 (win/high-frequency vs. loss/low-frequency trials) ANOVA. Only the results for main variables of interest, MAD and AUC, are reported here. The main effect of experiment was significant for MAD, F(2, 48) = 8.31, p < .01, η p 2 = .257, and AUC, F(2, 48) = 15.13, p < .001, η p 2 = .387. The main effect of trial type was significant for MAD, F(1, 48) = 26.29, p < .001, η p 2 = .354, and for AUC, F(1, 48) = 19.51, p < .001, η p 2 = .289. Furthermore, the interaction between experiment and trial type was significant for MAD, F(2, 48) = 5.37, p < .01, η p 2 = .183, and AUC, F(2, 48) = 3.88, p < .05, η p 2 = .139.
Planned comparisons revealed that the effect of trial type on the movement trajectories in the conflict condition of Experiment 1 and Experiment 2 were not different for MAD, t(48) = 1.49, p > .05, or for AUC, t(48) = 1.42, p > .05. However, both effects differed significantly from those in the control condition of Experiment 1 in MAD, F(1, 48) = 2.89, p < .01, and in AUC, F(1, 48) = 2.36, p < .05.
Discussion
Experiment 2 replicated the effect of self-control conflict in movement execution without presentation of emotional faces in the target areas and without feedback of ascending/descending tones. This finding rules out an alternative explanation in terms of a bias processing of emotional stimuli. In addition, it eliminates a potential confound with the affective quality of the sounds (see Horstmann, 2010). Instead, the results support the idea that the movement trajectories were influenced by motivational impulses to approach rewards (win) and/or avoid punishments (loss).
Please note that, unexpectedly, MAD and AUC were, in general, larger in Experiment 2 than in Experiment 1. Yet these between-experiment differences did not affect the crucial within manipulation of trial type, because in both conflict conditions, win and loss trials affected both measures similarly.
General discussion
This study examined an influence of self-control conflicts on action execution. To reveal the process of dynamic competition between goal-directed actions and temptations in movement execution, we recorded continuous mouse trajectories toward small rewards (point win) and punishments (loss of points). Two experiments provided clear evidence that the movement trajectories were deflected more when punishments were approached than when rewards were approached, indicating a self-regulatory conflict when an unattractive goal was pursued.
The present findings fit well with the general idea of two motivational systems of approach and avoidance that automatically direct behavior toward rewards and away from punishments (Eder, Elliot, & Harmon-Jones, 2013). In the present research, both motivations may have contributed to the observed effects, inducing different types of self-regulatory conflict on the loss trials: (1) a conflict between an automatic impulse to avoid the loss target and the intention to avoid an even greater loss by moving the manikin to this target (avoidance–avoidance conflict) and (2) a conflict between an automatic impulse to approach the win target and the intention to move the manikin to the loss target in line with the task instructions (approach–avoidance conflict). Furthermore, it is possible that approach and avoidance tendencies affected the movement trajectory simultaneously. Further research is necessary to determine whether the deflection in the movement trajectories on the loss trials was caused by an approach tendency, an avoidance tendency, or by a synergy of both motivations.
Theories of self-control are typically concerned with decision-making processes, and behavior is typically analyzed in terms of the achieved results (e.g., Friese & Hofmann, 2009). The means by which these goals are achieved (i.e., action execution) are, however, often overlooked. By quantifying action dynamics, we were able to demonstrate a self-control conflict not only in RTs and error rates, but also in the execution of a movement. Thus, a tug-of-war between antagonistic action tendencies appears to leak into action execution—a finding that is in line with dynamic action models that assume a constant accumulation of information and ongoing decision process until the behavior is terminated (Spivey, 2008).
Perhaps it is also interesting to interpret the present results from the reverse side. Not only does conflict affect movement execution, but also, conversely, the observation of movement execution might reveal something about the actor’s conflicts. In human–machine interaction, for example, it is of increasing importance to understand the user’s states and intentions. Carefully analyzing how a user moves a computer mouse (e.g., between potential buttons on a menu screen) might reveal something about his hidden interests and intentions that the mere recording of button clicks (i.e., choices) cannot reveal.
References
Baumeister, R. F. (2002). Yielding to temptation: Self–control failure, impulsive purchasing, and consumer behavior. Journal of Consumer Research, 28(4), 670–676.
Eder, A. B., Elliot, A. J., & Harmon-Jones, E. (2013). Approach and avoidance motivation: Issues and advances. Emotion Review, 5(3), 227–229.
Erlhagen, W., & Schöner, G. (2002). Dynamic field theory of movement preparation. Psychological Review, 109(3), 545–572.
Freeman, J. B., & Ambady, N. (2009). Motions of the hand expose the partial and parallel activation of stereotypes. Psychological Science, 20(10), 1183–1188.
Freeman, J. B., & Ambady, N. (2011). When two become one: Temporally dynamic integration of the face and voice. Journal of Experimental Social Psychology, 47(1), 259–263.
Freeman, J. B., Ambady, N., Rule, N. O., & Johnson, K. L. (2008). Will a category cue attract you? Motor output reveals dynamic competition across person construal. Journal of Experimental Psychology: General, 137(4), 673–690.
Friese, M., & Hofmann, W. (2009). Control me or I will control you: Impulses, trait self-control, and the guidance of behavior. Journal of Research in Personality, 43(5), 795–805.
Hofmann, W., Friese, M., & Strack, F. (2009). Impulse and self-control from a dual-systems perspective. Perspectives on Psychological Science, 4(2), 162–176.
Horstmann, G. (2010). Tone–affect compatibility with affective stimuli and affective responses. The Quarterly Journal of Experimental Psychology, 63(11), 2239–2250.
Metcalfe, J., & Mischel, W. (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review, 106(1), 3–19.
Miller, J. (1988). Discrete and continuous models of human information processing: Theoretical distinctions and empirical results. Acta Psychologica, 67(3), 191–257.
Muraven, M., & Baumeister, R. F. (2000). Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin, 126(2), 247.
Pfister, R., & Janczyk, M. (2013). Confidence intervals for two sample means: Calculation, of interpretation, and a few simple rules. Advances in Cognitive Psychology, 9(2), 74–80.
Schultz, W. (2006). Behavioral theories and the neurophysiology of reward. Annual Review of Psychology, 57, 87–115.
Spivey, M. (2008). The continuity of mind (Vol. 40). USA: Oxford University Press.
Spivey, M. J., Grosjean, M., & Knoblich, G. (2005). Continuous attraction toward phonological competitors. Proceedings of the National Academy of Sciences of the United States of America, 102(29), 10393–10398.
Sternberg, S. (1969). The discovery of processing stages: Extensions of Donders’ method. Acta Psychologica, 30, 276–315.
Strack, F., & Deutsch, R. (2004). Reflective and impulsive determinants of social behavior. Personality and Social Psychology Review, 8(3), 220–247.
Trope, Y., & Fishbach, A. (2000). Counteractive self-control in overcoming temptation. Journal of Personality and Social Psychology, 79(4), 493–506.
Author Note
This research was supported by a grant from the German Research Foundation (DFG) to Andreas Eder (ED 201/2-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dignath, D., Pfister, R., Eder, A.B. et al. Something in the way she moves—movement trajectories reveal dynamics of self-control. Psychon Bull Rev 21, 809–816 (2014). https://doi.org/10.3758/s13423-013-0517-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13423-013-0517-x