Abstract
Recent evidence has suggested that posttraumatic stress disorder (PTSD) is associated with alterations in prefrontal-cortex-dependent cognitive processes (e.g., working memory, cognitive control). However, it remains unclear whether these cognitive dysfunctions are related to PTSD symptomatology or trauma exposure. Furthermore, regarding cognitive control, research has only focused on the integrity of selected control functions, but not their dynamic regulation in response to changing environmental demands. Therefore, the present study investigated dynamic variations in interference control, in addition to overall interference susceptibility and working memory (WM) performance in matched groups of 24 PTSD patients and 26 traumatized and 30 nontraumatized healthy controls. The Simon task was used to measure overall interference susceptibility and the flexible adjustment of cognitive control, on the basis of the occurrence of response conflicts (conflict adaptation effect). WM performance was assessed with the forward and backward digit span tasks. Since we have previously shown that trauma exposure per se is associated with reduced hair cortisol concentrations (HCC), we further explored whether PTSD/trauma-related cognitive alterations are related to HCC in proximal 3-cm hair segments. The results revealed that PTSD patients and traumatized controls showed significantly more pronounced conflict adaptation effects than nontraumatized controls. Moreover, the conflict adaptation effect was positively related to the number of lifetime traumatic events and the frequency of traumatization. The groups did not differ in overall interference susceptibility or WM performance. Exploratory analyses revealed no association between HCC and the observed cognitive differences. These results suggest that context-driven control adjustments constitute a sensitive correlate of trauma exposure, irrespective of PTSD.
Similar content being viewed by others
Posttraumatic stress disorder (PTSD) emerges as a possible clinical outcome from exposure to one or more traumatic events and involves cognitive impairments, including the involuntary recollection of the traumatic event, the inability to recall important aspects of the trauma, and concentration difficulties (American Psychiatric Association, 2000). Previous research has frequently examined neuropsychological alterations associated with PTSD, with a particular focus on hippocampal-dependent memory impairments. Another important aspect of cognitive functioning relates to prefrontal cortex (pFC) dependent processes that are crucially involved in the intentional manipulation of traumatic memories (Depue, Curran, & Banich, 2007). The pFC represents the main neural correlate of working memory (WM), as well as of the cognitive-control processes crucial for goal-directed behavior and adaptive responding to challenging and novel conditions (Miller & Cohen, 2001).
Indeed, PTSD patients are assumed to exhibit structural and functional changes of the pFC (reviewed in Pitman et al., 2012) and impaired pFC-dependent functions (reviewed in Aupperle, Melrose, Stein, & Paulus, 2012; Polak, Witteveen, Reitsma, & Olff, 2012). More specifically, the majority of studies have detected poorer WM performance in PTSD patients in comparison to traumatized controls (e.g., Jelinek et al., 2008; Koso & Hansen, 2006; Vasterling et al., 2002) or both traumatized and nontraumatized controls (Jenkins, Langlais, Delis, & Cohen, 2000; Lagarde, Doyon, & Brunet, 2010). Although these findings suggest that WM deficits are related to PTSD, there is also evidence for a general impairment in trauma-exposed groups, irrespective of PTSD (e.g., El-Hage, Gaillard, Isingrini, & Belzung, 2006). However, a number of studies have also failed to find differences between such groups (e.g., Burriss, Ayers, Ginsberg, & Powell, 2008; Moores et al., 2008; Twamley, Hami, & Stein, 2004). Another set of studies has focused on response inhibition as a key cognitive control function that prevents prepotent but unwanted responses from being executed (Miyake et al., 2000). These revealed decreased response inhibition in PTSD patients relative to traumatized controls (dichotic-listening task: Johnsen, Kanagaratnam, & Asbjornsen, 2011; attentional network task: Leskin & White, 2007; go–no-go task: Wu et al., 2010) or nontraumatized controls (go–no-go task: Falconer et al., 2008). Furthermore, previous research examined PTSD- or trauma-related differences in interference susceptibility by using conventional Stroop tasks, in which participants name the color of a word, whereas the word itself can either correspond with the ink color (e.g., “red” written in red) or not (e.g., “green” written in red). This results in an automatic activation of the corresponding response that facilitates task performance or the noncorresponding response that interferes with the instructed task, respectively. Interestingly, the majority of these studies have revealed no differences between PTSD patients and controls (e.g., Eren-Kocak, Kilic, Aydin, & Hizli, 2009; Lindauer, Olff, van Meijel, Carlier, & Gersons, 2006; Litz et al., 1996; Vasterling et al., 2002). The picture, however, is quite different in Stroop versions using emotionally negative, threat- or trauma-related stimuli. Here, interference was found to be increased in PTSD patients (e.g., Litz et al., 1996; Mueller-Pfeiffer et al., 2010; Shin et al., 2001; Vythilingam et al., 2007) and to be negatively related to intrusion symptoms (e.g., Paunovi, Lundh, & Ost, 2002). Interestingly, recent meta-analytic data have indicated that an increased emotional Stroop interference is also found in traumatized individuals without PTSD (Cisler et al., 2011). Whereas these findings suggest a PTSD/trauma-related attentional bias toward negative affective stimuli (reviewed in Hayes, Vanelzakker, & Shin, 2012), it still remains unclear whether pFC-dependent functions also extend beyond trauma-related cognitive impairments.
Additionally, whereas most previous studies have solely investigated the integrity of singular cognitive control functions (e.g., response inhibition) in PTSD patients, the ability to dynamically regulate cognitive control in response to changing situational demands has not been addressed, yet. This, however, seems to be particularly important as everyday life requires successful performance in fast changing high-demanding environments. In recent years, this flexible adjustment of cognitive control has been frequently investigated by assessing changes in interference effects following response conflicts. The most widely used paradigm for this is the Simon task, in which participants categorize stimuli on the basis of their identity (e.g., single-digit numbers as smaller or larger than five) by pressing a left or right response button. The stimuli appear either to the left or right of the screen center. Although stimulus location is completely task-irrelevant, it automatically activates the spatially corresponding response, which can either be in accordance with the required one (compatible condition) or differ from it (incompatible condition), with the latter causing a response conflict that needs to be inhibited. The difference between the compatible and incompatible condition denotes the Simon effect as measure of interference susceptibility and response inhibition. Additionally, experience of a response conflict typically reduces interference susceptibility in the subsequent trial in comparison to trials following a nonconflict (reviewed in Botvinick, Cohen, & Carter, 2004). This interaction effect between previous and current trial types is referred to as conflict adaptation effect and assumed to result from flexible adjustments of cognitive control in response to conflict. Recent research has shown that this conflict adaptation effect represents a sensitive measure of interindividual (e.g., Keye, Wilhelm, Oberauer, & van Ravenzwaaij, 2009; Krug & Carter, 2010) and developmental (Waxer & Morton, 2011) differences in conflict processing and adaptation, and is modulated by affect or reward (e.g., Braem, Verguts, Roggeman, & Notebaert, 2012; van Steenbergen, Band, & Hommel, 2009, 2010) and by stress (Plessow, Fischer, Kirschbaum, & Goschke, 2011).
Based on previous research, it remains unclear whether potential alterations in cognitive control are related to PTSD symptomatology, or rather to trauma exposure. Thus, the present study examined groups of (1) PTSD patients, (2) traumatized healthy controls (TC), and (3) nontraumatized healthy controls (NTC; as previously reported in Steudte et al., 2013) regarding WM performance, interference susceptibility and its sequential modulation. Furthermore, we examined whether the assessed cognitive parameters are associated with trauma-related characteristics and PTSD symptom patterns. Interestingly, we have previously found lower long-term integrated hair cortisol concentrations (HCC) in traumatized individuals (with and without PTSD) and negative HCC relationships with trauma- and symptom-related aspects (Steudte et al., 2013). Since cortisol is known to exert potent effects on pFC-dependent processes (reviewed in Arnsten, 2009), we further explored whether potential PTSD/trauma-related alterations in pFC-dependent functions are related to lower HCC. Importantly, our previous research shows that acute cortisol stress reactivity is negatively associated with the amount of conflict adaptation (Plessow et al., 2011). Combining this with our recent finding that PTSD patients and traumatized controls exhibit attenuated long-term cortisol levels (Steudte et al., 2013) makes it conceivable that these two groups also show altered flexible control adjustments, as compared to nontraumatized controls.
Method
Participants
Participants were recruited via flyers and local advertisements. PTSD patients were additionally enrolled from the outpatient unit of the Institute of Clinical Psychology and Psychotherapy of the Technische Universität Dresden (Steudte et al., 2013). Participants were excluded if they had hair shorter than 3 cm at the posterior vertex region of the scalp, signs of baldness or hair loss, were pregnant, reported suffering from any severe physical disease over the past 5 years and/or the use of glucocorticoid-containing medicine, or psychotropic medications (e.g., antidepressants) within the past six months.
Diagnostic assessments for DSM-IV mental disorders were based on the standardized Munich Composite International Diagnostic Interview (DIA-X/M-CIDI; Wittchen & Pfister, 1997). PTSD patients met the criteria of a current primary 12-month PTSD diagnosis and had no 12-month diagnosis of substance abuse or dependence (except for nicotine), lifetime diagnoses of bipolar disorder, psychosis and/or severe depressive disorder with psychotic symptoms. None of the control participants (TC, NTC) had a psychiatric lifetime disorder as assessed with the stem questions of the DIA-X/M-CIDI and the Mini International Neuropsychiatric Interview (M.I.N.I.; Sheehan et al., 1998). Assignment to both control groups was based on the items of the trauma checklist of the Posttraumatic Diagnostic Scale (PDS; Ehlers, Steil, Winter, & Foa, 1996): TC participants had to have experienced a traumatic event according to the A1 and A2 criterion of DSM-IV, whereas this was not the case for the NTC group.
24 PTSD patients, 26 TC and 30 NTC participants from the sample described in Steudte et al. (2013) took part in the neuropsychological examination. The groups were matched regarding age, sex, body mass index (BMI), smoking status, and use of oral contraceptives. Table 1 provides detailed descriptive information (including HCC) for the three groups. Both groups of traumatized individuals had experienced civilian traumatic events comprising accidents (n = 9), natural disasters (n = 6), sexual assaults (n = 12), physical assaults (n = 8), life-threatening illnesses (n = 5), and other traumatic events (e.g., the sudden death of a close relative; n = 10). Of the trauma-exposed individuals, 74 % had experienced the relevant traumatic event more than five years ago. Comorbid diagnoses of PTSD patients included depressive disorders (n = 16), specific phobia (n = 8), social phobia (n = 4), pain disorder (n = 3), generalized anxiety disorder (n = 4), panic disorder with or without agoraphobia (n = 3), and obsessive-compulsive disorder (n = 1).
All participants provided written informed consent prior to taking part in the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Technische Universität Dresden Medical School.
Clinical and psychological measures
Sociodemographic information (sex, age, educational status, BMI, smoking status, use of oral contraceptives, menopausal status), hair-specific characteristics (washes per week, curls or waves, hair treatments, and natural hair color), and participants’ health (medication intake, physical diseases) were recorded using a self-developed questionnaire. The use of hair treatments was defined as the use of either semipermanent or permanent coloration, or permanent wave or hair straightening during the past three months (as in Stalder et al., 2012). The PDS (Ehlers et al., 1996) was used to measure the severity of overall PTSD symptoms over the last month, whereas the Impact of Event Scale–Revised (IES-R; Maercker & Schützewohl, 1998) was utilized to assess the severity of PTSD symptom clusters (intrusions, avoidance, hyperarousal) over the last week. The Childhood Trauma Questionnaire (CTQ; Wingenfeld et al., 2010) was applied to measure childhood maltreatment, and the Trauma History Questionnaire (THQ; Maercker, 2002) was further used to provide a measure of lifetime exposure to traumatic events (according to the A1 criterion). The THQ items assess lifetime exposure to 24 different traumatic events (0 = no, 1 = yes) and the number of occurrences for each event (0 = never, 1 = once, 2 = 2 times, 3 = 3 times, 4 = 4 times, 5 = 5 times, 6 = 6 or more times). The respective sum scores reflect (1) the number of different lifetime traumatic events and (2) the frequency of traumatization. The Beck Depression Inventory (BDI-II; Hautzinger, Keller, & Kühner, 2006) was used to quantify the severity of depressive symptoms, whereas the Screening Scale of Chronic Stress of the Trier Inventory for the Assessment of Chronic Stress (SSCS-TICS; Schulz, Schlotz, & Becker, 2004) was obtained to assess chronic stress over the last three months.
Cognitive tasks
Simon task
Interference susceptibility and conflict adaptation were assessed via a number version of the classical Simon task (Fischer, Dreisbach, & Goschke, 2008). Participants categorized single-digit numbers (1–9, except 5) as smaller or larger than five by pressing a left (Alt key) or a right (Alt-Gr key) response key on a QWERTZ keyboard with their left or right index finger, respectively. The numbers were presented either to the left or to the right of the screen center, resulting in compatible trials (C; e.g., “1” presented on the left location) or incompatible trials (I; e.g., “1” displayed on the right location). Using an IBM-compatible personal computer with presentation software (version 0.71; Neurobehavioral systems, Inc., Albany, CA, USA), target stimuli were presented white against black on a 17-in. monitor. Viewing distance was approximately 60 cm. Trial started with a central presentation of a fixation cross. After 1,000 ms, targets (0.48º–0.67º) were shown either 2.8 cm to the left or to the right of the fixation sign (visual angle of 2.7° to the left and right) for 200 ms. Once a response was given (max. of 1,800 ms from target onset), participants received performance feedback together with a sinus tone through headphones: For correct responses, a blank screen (300 ms) was shown, for incorrect responses or misses “false” or “too slow” were presented, respectively, followed by a blank screen for a random interval between 100 and 1,000 ms. Participants performed a practice block (16 trials) and three test blocks (64 trials each = total number of test trials: 192). The numerical variant of the Simon task was chosen over classical versions of the paradigm, as it features more than one stimulus for each response alternative. This allowed for the exclusion of identical target repetitions, minimizing the impact of feature binding processes on task performance (Mayr, Awh, & Laurey, 2003).
Forward and backward digit span task
WM performance was measured using the forward and backward digit span tasks of the Wechsler Memory Scale (WMS-III; Wechsler, 1997). The forward digit span task was used to assess the maintenance of information, whereas the backward digit span task was obtained to measure the manipulation of information. The total digit span is based on the sum score of the forward and backward digit spans providing a measure of overall WM performance.
Hair cortisol analysis
Hair strands (~3mm diameter) were taken near the scalp from a posterior vertex position, and cortisol concentrations in the proximal 3-cm hair segment were measured. HCC in the first 3-cm hair segment are assumed to reflect integrated cortisol secretion over the 3-month period prior to hair sampling (see Stalder & Kirschbaum, 2012). HCC were determined via liquid chromatography tandem mass spectrometry. Detailed information on the analytical protocol is provided in Gao et al. (2013).
Statistical analysis
Group comparisons regarding sociodemographic, hair-related, and clinical/psychological characteristics were conducted using χ 2 contingency tables (for dichotomous variables) or univariate analyses of variance (ANOVAs, for continuous variables). Measures of PTSD symptoms were compared between the TC and PTSD groups by applying t tests. For the Simon task, three-way mixed-design ANOVAs with Compatibility of the Current Trial (2 levels: compatible vs. incompatible) and Compatibility of the Previous Trial (2 levels: compatible vs. incompatible) as within-subjects factors, and Group (3 levels: PTSD, TC, NTC) as a between-subjects factor, were conducted for median reaction times (RTs) and error rates, respectively. For this analysis, the first trial of each block (1.19 %), posterror trials (2.97 %), and trials with identical target repetitions (10.12 %) were excluded. Furthermore, for the analysis of RTs, error trials were omitted (2.48 %). The analyses were repeated by adding BDI-II, CTQ, and SSCS-TICS scores as covariates, to control for potential confounding influences. Furthermore, indices of overall interference susceptibility (I – C) and conflict adaptation [(cI – cC) – (iI – iC), with lowercase letters denoting the compatibility in the previous trial and capitals the compatibility in the current trial] were calculated (see also van Steenbergen et al., 2010), with larger indices indicating more interference susceptibility and more flexible adaptation to changing environmental demands, respectively. Group differences in performance of the digit span tasks were examined using univariate ANOVAs. Using Spearman correlations, associations were examined between selective cognitive outcome parameters and (1) trauma-related variables, (2) HCC, and (3) the severity of PTSD symptoms (in traumatized groups only).
Results
Sample characteristics
The groups were well matched on the sociodemographic and hair-related variables (ps > .09; see Table 1). Furthermore, the groups differed on measures of depressive symptoms (BDI-II), maltreatment in childhood (CTQ), the number and frequency (THQ) of traumatization, and perceived chronic stress (SSCS-TICS; ps < .001). Bonferroni post-hoc analyses revealed that PTSD patients scored higher on BDI-II, SCCS-TICS, and frequency of traumatization than both TC (ps < .05) and NTC (ps < .01) participants. Traumatized groups (PTSD, TC) reported having experienced a larger number of different A1 traumatic events and scored higher on CTQ scores than NTC controls (ps < .05) and did not differ from each other in these measures (ps > .116). As expected, PTSD patients exhibited greater symptom severity than TC participants (PDS sum score, IES-R subscales; ps < .001).
Simon task
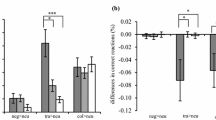
Median RTs and percentages of errors are presented in Table 2. The RT analysis revealed a main effect of compatibility (incompatible, 565 ms; compatible, 541 ms) [F(1, 77) = 79.30, p < .001, η p 2 = .507]. Furthermore, an interaction between current and previous trial compatibility occurred, in which interference effects were reduced following conflict trials (–3 ms) in comparison to nonconflict trials (55 ms), reflecting the typical conflict adaptation effect [F(1, 77) = 266.47, p < .001, η p 2 = .776]. Whereas the overall interference effect did not differ between groups [F(2, 77) = 0.63, p = .534, η p 2 = .016], a group difference in conflict adaptation was found [F(2, 77) = 6.33, p < .01, η p 2 = .141]. Figure 1 illustrates the conflict adaptation effects for the three groups. Bonferroni post-hoc comparisons of the conflict adaptation index revealed that both PTSD patients and TC controls showed a higher conflict adaptation effect than NTC controls (both ps < .05), with no differences between the PTSD and TC groups (p = 1.00). Including CTQ, BDI-II, and SSCS scores as covariates revealed no significant effects (p > .205). Furthermore, a main effect of group on overall RTs was revealed [F(2, 77) = 3.72, p = .029, η p 2 = .088]. Bonferroni post-hoc analyses indicated that PTSD patients had longer RTs than NTC participants (p = .042), with a statistical trend relative to TC controls (p = .084) and no differences between the TC and NTC groups (p = 1.00). Importantly, between-group differences in conflict adaptation remained significant when repeating the main analysis including individual mean median RTs as a covariate [F(2, 76) = 4.78, p < .05, η p 2 = .112]. Exploratory analyses revealed no association between RT conflict adaptation and HCC (r S = –.098, p = .385).
Our analysis of error rates also showed main effects of compatibility (incompatible, 3.77 %; compatible, 2.18 %) [F(1, 77) = 14.20, p < .001, η p 2 = .156] and overall conflict adaptation (–1.01 % vs. 4.03 %) [F(1, 77) = 56.32, p < .001, η p 2 = .422]. Neither a significant main effect of group [F(2, 77) = 0.47, p = .626, η p 2 = .012] nor an interaction with compatibility or conflict adaptation (ps > .82) was found. Importantly, the pattern of error rate data does not contradict the pattern of RT findings, which rules out the possibility of a speed–accuracy trade-off (see Table 2).
Correlation analyses revealed a positive association between the conflict adaptation effects in median RTs and the number of different lifetime traumatic events (r S = .228, p = .042) and the frequency of traumatization (r S = .252, p = .025) (shown in Fig. 2). Except for a trend toward a positive relationship with the severity of avoidance symptoms (r S = .276, p = .052), no associations between conflict adaptation and PTSD symptom severity were observed (ps > .361). Positive relationships were further found between RT conflict adaptation and BDI-II scores (r S = .253, p = .024) and CTQ scores (r S = .290, p < .01).
Forward and backward digit span tasks
No significant group differences were observed on the three measures of WM performance (ps > .325; see Table 3). In traumatized individuals, a negative association between the forward digit span and intrusion symptoms (r S = –.377, p < .01) and a trend toward a negative relationship with avoidance symptoms (r S = –.259, p = .069) were found. No further WM relationships with PTSD symptoms were detected (ps > .107).
Discussion
To our knowledge, this is the first study demonstrating that trauma exposure is a crucial correlate of flexible control adjustments, irrespective of PTSD, as evidenced by a higher conflict adaptation in PTSD patients and traumatized healthy controls than in nonexposed controls. Furthermore, more pronounced cognitive control adjustments were related to a larger number and higher frequency of traumatization. In contrast to substantial differences in conflict adaptation, no group differences were observed in overall interference susceptibility or WM performance. Exploratory analyses revealed no relationship between conflict adaptation and HCC.
The central finding of the present study was that both PTSD patients and traumatized healthy controls present higher conflict-driven control adjustments. This is the first demonstration of trauma-related changes in the ability to dynamically regulate the involvement of cognitive control in response to changing environmental demands. By contrast, no differences in overall interference susceptibility from a task-irrelevant stimulus feature were found, which concurs with most previous studies using the classical Stroop paradigm (e.g., Eren-Kocak et al., 2009; Lindauer et al., 2006; Vasterling et al., 2002). This presence of a trial-to-trial effect in the absence of an effect on overall interference susceptibility has been previously reported in the literature (e.g., Liepelt, Wenke, Fischer, & Prinz, 2011; Plessow et al., 2011) and highlights the importance of using more sensitive measures of dynamic situation-dependent control adjustments to unveil effects that might otherwise be masked (e.g., by averaging each other out).
Starting from the assumption that PTSD patients and/or trauma-exposed individuals might exhibit impaired pFC-dependent functions, the present finding of increased control adjustments in trauma-exposed individuals may seem surprising. However, for the interpretation of the current results, it is noteworthy that recent studies have provided evidence for the generally aversive nature of response conflicts per se (e.g., Dreisbach & Fischer, 2012). Thus, it is conceivable that traumatized groups are more sensitive to response conflicts, which then results in a more pronounced conflict adaptation effect, given that it has been reported that they also show enhanced detection and/or processing of trauma-related stimuli (Cisler et al., 2011). The assumption of an enhanced conflict processing in trauma-exposed individuals gets further support from recent findings suggesting a hyperactive dorsal anterior cingulated cortex (dACC) in PTSD patients (reviewed in Hayes, Hayes, & Mikedis, 2012; Pitman et al., 2012)—an area known to be particularly important for the detection of response conflicts and for signaling the need for strategic adjustments in cognitive control to the dorsolateral pFC (reviewed in Botvinick et al., 2004). Interestingly, increased ACC activation was found to be related to a more pronounced conflict adaptation (van Steenbergen, Band, Hommel, Rombouts, & Nieuwenhuis, 2014). A hyperactive dACC may thus account for enhanced cognitive control adjustments in PTSD patients, albeit it is again not clear whether this is PTSD-specific or more generally trauma-related. As proposed for individuals with high trait anxiety, trauma-exposed groups may use a reactive control strategy, which is assumed to enable an optimal detection of environmental changes and shift of attention to distracters (Braver, Gray, & Burgess, 2007; Krug & Carter, 2010). Therefore, an enhanced conflict adaptation may constitute an adaptive mechanism allowing an improved monitoring of potentially threatening stimuli as well as an immediate response to them in the environment. In addition, our data revealed a reduced overall processing speed specifically in PTSD patients. Although this is in line with most previous research (e.g., Kanagaratnam & Asbjornsen, 2007; Twamley et al., 2009), it is noteworthy that this effect is independent of the present conflict adaptation findings.
The crucial importance of trauma exposure on control adjustments was further supported by the present findings of positive associations between the amount of conflict adaptation and number and frequency of traumatization. Interestingly, this pattern of relationships is in line with the notion of a building block effect—that is, the impact of trauma exposure on the incidence of PTSD in a dose-response manner—which is assumed to be mediated through sensitization of the neural fear network (Kolassa & Elbert, 2007). It is conceivable that this building block effect could be mirrored in the present conflict adaptation findings, in the form of a cumulative learning experience.
The pattern of altered cognitive control adjustments in traumatized individuals, irrespective of PTSD, closely corresponds with our finding of altered long-term cortisol secretion in these groups (Steudte et al., 2013). This together with the fact that associations with number and frequency of traumatization have been revealed for both HCC and conflict adaptation support the assumption of a potential link between hypothalamic-pituitary-adrenal axis alterations and cognitive control adjustments. Furthermore, the finding of a higher conflict adaptation effect for both traumatized groups—which are characterized by lower cortisol levels—fits well with recent observations of less flexible control adjustments in conditions of experimentally increased salivary cortisol levels in reaction to acute stress (Plessow et al., 2011). However, it is important to note that we found no significant association between HCC and conflict adaptation on a within-group level. Given the relatively small sample size and large inter-individual variance, this may be the result of insufficient statistical power to detect a meaningful relationship between these variables. Further research is required to investigate the potential link between long-term endocrine correlates and conflict adaptation in larger samples.
With regard to WM performance, we found no evidence for trauma- or PTSD-related deficits. Although this is in line with previous research (e.g., Burriss et al., 2008; Moores et al., 2008; Twamley et al., 2004), it is at variance with a number of studies suggesting deficient WM performance in PTSD patients or trauma-exposed individuals (e.g., El-Hage et al., 2006; Jenkins et al., 2000; Vasterling et al., 2002). Nonetheless, a reduced ability to maintain information was found to be related to more severe intrusion symptoms in traumatized groups, which is in line with a recent study (Bomyea, Amir, & Lang, 2012).
Some limitations of the present study consist of the small sample size and the high proportion of comorbid major depressive disorders in the PTSD group. Regarding depressive symptoms, we revealed a positive relationship with conflict adaptation, which was recently also found in remitted depressive patients (van Steenbergen, Booij, Band, Hommel, & van der Does, 2012). Importantly, since traumatized controls (without major depression) also revealed a higher conflict adaptation and controlling for BDI-II scores did not change the respective results, depressive symptomatology seems not to explain the main study findings. In addition, conflict adaptation was positively related to childhood maltreatment. This together with the fact that both traumatized groups experienced more severe childhood maltreatment as compared to nontraumatized controls makes it conceivable that early adverse experiences might lead to durable changes in cognitive control adjustments. However, controlling for CTQ scores did not change the present pattern of results.
The current findings suggest that trauma exposure constitutes a crucial correlate of flexible control adjustments, even in the absence of psychopathology, which warrants further investigation. The fact that trauma-related alterations were not seen in overall interference susceptibility or WM performance highlights the importance of the analysis of dynamic situation-dependent control adjustments for future clinical research.
References
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders, text revision (DSM-IV-TR) (4th ed.). Washington, DC: American Psychiatric Press.
Arnsten, A. F. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–422. doi:10.1038/nrn2648
Aupperle, R. L., Melrose, A. J., Stein, M. B., & Paulus, M. P. (2012). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62, 686–694. doi:10.1016/j.neuropharm.2011.02.008
Bomyea, J., Amir, N., & Lang, A. J. (2012). The relationship between cognitive control and posttraumatic stress symptoms. Journal of Behavior Therapy and Experimental Psychiatry, 43, 844–848. doi:10.1016/j.jbtep.2011.12.001
Botvinick, M. M., Cohen, J. D., & Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8, 539–546. doi:10.1016/j.tics.2004.10.003
Braem, S., Verguts, T., Roggeman, C., & Notebaert, W. (2012). Reward modulates adaptations to conflict. Cognition, 125, 324–332. doi:10.1016/j.cognition.2012.07.015
Braver, T. S., Gray, J. R., & Burgess, G. C. (2007). Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. In A. R. A. Conway, C. Jarrold, M. J. Kane, A. Miyake, & J. N. Towse (Eds.), Variation in working memory (pp. 76–106). Oxford, UK: Oxford University Press.
Burriss, L., Ayers, E., Ginsberg, J., & Powell, D. A. (2008). Learning and memory impairment in PTSD: Relationship to depression. Depression and Anxiety, 25, 149–157. doi:10.1002/da.20291
Cisler, J. M., Wolitzky-Taylor, K. B., Adams, T. G., Jr., Babson, K. A., Badour, C. L., & Willems, J. L. (2011). The emotional Stroop task and posttraumatic stress disorder: A meta-analysis. Clinical Psychology Review, 31, 817–828. doi:10.1016/j.cpr.2011.03.007
Depue, B. E., Curran, T., & Banich, M. T. (2007). Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science, 317, 215–219. doi:10.1126/science.1139560
Dreisbach, G., & Fischer, R. (2012). Conflicts as aversive signals. Brain and Cognition, 78, 94–98. doi:10.1016/j.bandc.2011.12.003
Ehlers, A., Steil, R., Winter, H., & Foa, E. B. (1996). Deutsche Übersetzung der Posttraumatic Diagnostic Scale (PDS). Oxford, UK: Oxford University, Warneford Hospital.
El-Hage, W., Gaillard, P., Isingrini, M., & Belzung, C. (2006). Trauma-related deficits in working memory. Cognitive Neuropsychiatry, 11, 33–46. doi:10.1080/13546800444000164
Eren-Kocak, E., Kilic, C., Aydin, I., & Hizli, F. G. (2009). Memory and prefrontal functions in earthquake survivors: Differences between current and past post-traumatic stress disorder patients. Acta Psychiatrica Scandinavica, 119, 35–44. doi:10.1111/j.1600-0447.2008.01281.x
Falconer, E., Bryant, R., Felmingham, K. L., Kemp, A. H., Gordon, E., Peduto, A., & Williams, L. M. (2008). The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry and Neuroscience, 33, 413–422.
Fischer, R., Dreisbach, G., & Goschke, T. (2008). Context-sensitive adjustments of cognitive control: Conflict-adaptation effects are modulated by processing demands of the ongoing task. Journal of Experimental Psychology. Learning, Memory, and Cognition, 34, 712–718. doi:10.1037/0278-7393.34.3.712
Gao, W., Stalder, T., Foley, P., Rauh, M., Deng, H., & Kirschbaum, C. (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of Chromatography B, 928, 1–8. doi:10.1016/j.jchromb.2013.03.008
Hautzinger, M., Keller, F., & Kühner, C. (2006). Beck depressions-inventar (BDI-II) (revision). Frankfurt am Main, Germany: Harcourt Test Services.
Hayes, J. P., Hayes, S. M., & Mikedis, A. M. (2012a). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders, 2, 9. doi:10.1186/2045-5380-2-9
Hayes, J. P., Vanelzakker, M. B., & Shin, L. M. (2012b). Emotion and cognition interactions in PTSD: A review of neurocognitive and neuroimaging studies. Frontiers in Integrative Neuroscience, 6, 89. doi:10.3389/fnint.2012.00089
Jelinek, L., Moritz, S., Randjbar, S., Sommerfeldt, D., Puschel, K., & Seifert, D. (2008). Does the evocation of traumatic memories confound subsequent working memory performance in posttraumatic stress disorder (PTSD)? Depression and Anxiety, 25, 175–179. doi:10.1002/da.20300
Jenkins, M. A., Langlais, P. J., Delis, D. A., & Cohen, R. A. (2000). Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. Clinical Neuropsychologist, 14, 7–12. doi:10.1076/1385-4046(200002)14:1;1-8;FT007
Johnsen, G. E., Kanagaratnam, P., & Asbjornsen, A. E. (2011). Patients with posttraumatic stress disorder show decreased cognitive control: Evidence from dichotic listening. Journal of the International Neuropsychological Society, 17, 344–353. doi:10.1017/S1355617710001736
Kanagaratnam, P., & Asbjornsen, A. E. (2007). Executive deficits in chronic PTSD related to political violence. Journal of Anxiety Disorders, 21, 510–525. doi:10.1016/j.janxdis.2006.06.008
Keye, D., Wilhelm, O., Oberauer, K., & van Ravenzwaaij, D. (2009). Individual differences in conflict-monitoring: Testing means and covariance hypothesis about the Simon and the Eriksen flanker task. Psychological Research, 73, 762–776. doi:10.1007/s00426-008-0188-9
Kolassa, I.-T., & Elbert, T. (2007). Structural and functional neuroplasticity in relation to traumatic stress. Current Directions in Psychological Science, 16, 321–325. doi:10.1111/j.1467-8721.2007.00529.x
Koso, M., & Hansen, S. (2006). Executive function and memory in posttraumatic stress disorder: A study of Bosnian war veterans. European Psychiatry, 21, 167–173. doi:10.1016/j.eurpsy.2005.06.004
Krug, M. K., & Carter, C. S. (2010). Adding fear to conflict: A general purpose cognitive control network is modulated by trait anxiety. Cognitive, Affective, & Behavioral Neuroscience, 10, 357–371. doi:10.3758/CABN.10.3.357
Lagarde, G., Doyon, J., & Brunet, A. (2010). Memory and executive dysfunctions associated with acute posttraumatic stress disorder. Psychiatry Research, 177, 144–149. doi:10.1016/j.psychres.2009.02.002
Leskin, L. P., & White, P. M. (2007). Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology, 21, 275–284. doi:10.1037/0894-4105.21.3.275
Liepelt, R., Wenke, D., Fischer, R., & Prinz, W. (2011). Trial-to-trial sequential dependencies in a social and non-social Simon task. Psychological Research, 75, 366–375. doi:10.1007/s00426-010-0314-3
Lindauer, R. J., Olff, M., van Meijel, E. P., Carlier, I. V., & Gersons, B. P. (2006). Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biological Psychiatry, 59, 171–177. doi:10.1016/j.biopsych.2005.06.033
Litz, B. T., Weathers, F. W., Monaco, V., Herman, D. S., Wulfsohn, M., Marx, B., & Keane, T. M. (1996). Attention, arousal, and memory in posttraumatic stress disorder. Journal of Traumatic Stress, 9, 497–519. doi:10.1002/jts.2490090308
Maercker, A. (2002). THQ—Trauma history questionnaire—deutsche Fassung: Kurznachweis. Zürich, Switzerland: Universität Zürich.
Maercker, A., & Schützewohl, M. (1998). Erfassung von psychischen Belastungsfolgen: Die Impact of Event Skala—revidierte Version. Diagnostica, 44, 130–141.
Mayr, U., Awh, E., & Laurey, P. (2003). Conflict adaptation effects in the absence of executive control. Nature Neuroscience, 6, 450–452. doi:10.1038/nn1051
Miller, E. K., & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. doi:10.1146/annurev.neuro.24.1.167
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. doi:10.1006/cogp.1999.0734
Moores, K. A., Clark, C. R., McFarlane, A. C., Brown, G. C., Puce, A., & Taylor, D. J. (2008). Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Research, 163, 156–170. doi:10.1016/j.pscychresns.2007.08.011
Mueller-Pfeiffer, C., Martin-Soelch, C., Blair, J. R., Carnier, A., Kaiser, N., Rufer, M., & Hasler, G. (2010). Impact of emotion on cognition in trauma survivors: What is the role of posttraumatic stress disorder? Journal of Affective Disorders, 126, 287–292. doi:10.1016/j.jad.2010.03.006
Paunovi, N., Lundh, L. G., & Ost, L. G. (2002). Attentional and memory bias for emotional information in crime victims with acute posttraumatic stress disorder (PTSD). Journal of Anxiety Disorders, 16, 675–692. doi:10.1016/S0887-6185(02)00136-6
Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., & Liberzon, I. (2012). Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience, 13, 769–787. doi:10.1038/nrn3339
Plessow, F., Fischer, R., Kirschbaum, C., & Goschke, T. (2011). Inflexibly focused under stress: Acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. Journal of Cognitive Neuroscience, 23, 3218–3227. doi:10.1162/jocn_a_00024
Polak, A. R., Witteveen, A. B., Reitsma, J. B., & Olff, M. (2012). The role of executive function in posttraumatic stress disorder: A systematic review. Journal of Affective Disorders, 141, 11–21. doi:10.1016/j.jad.2012.01.001
Schulz, P., Schlotz, W., & Becker, P. (2004). Trierer Inventar zum chronischen Stress (TICS). Göttingen, Germany: Hogrefe.
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., & Dunbar, G. C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59(Suppl. 20), 22–33. quiz 34–57.
Shin, L. M., Whalen, P. J., Pitman, R. K., Bush, G., Macklin, M. L., Lasko, N. B., & Rauch, S. L. (2001). An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry, 50, 932–942. doi:10.1016/S0006-3223(01)01215-X
Stalder, T., & Kirschbaum, C. (2012). Analysis of cortisol in hair—State of the art and future directions. Brain, Behavior, and Immunity, 26, 1019–1029. doi:10.1016/j.bbi.2012.02.002
Stalder, T., Steudte, S., Alexander, N., Miller, R., Gao, W., Dettenborn, L., & Kirschbaum, C. (2012). Cortisol in hair, body mass index and stress-related measures. Biological Psychology, 90, 218–223. doi:10.1016/j.biopsycho.2012.03.010
Steudte, S., Kirschbaum, C., Gao, W., Alexander, N., Schönfeld, S., Hoyer, J., & Stalder, T. (2013). Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biological Psychiatry, 74, 639–646. doi:10.1016/j.biopsych.2013.03.011
Twamley, E. W., Allard, C. B., Thorp, S. R., Norman, S. B., Hami Cissell, S., Hughes Berardi, K., & Stein, M. B. (2009). Cognitive impairment and functioning in PTSD related to intimate partner violence. Journal of the International Neuropsychological Society, 15, 879–887. doi:10.1017/S135561770999049X
Twamley, E. W., Hami, S., & Stein, M. B. (2004). Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Research, 126, 265–274. doi:10.1016/j.psychres.2004.01.008
van Steenbergen, H., Band, G. P. H., & Hommel, B. (2009). Reward counteracts conflict adaptation. Evidence for a role of affect in executive control. Psychological Science, 20, 1473–1477. doi:10.1111/j.1467-9280.2009.02470.x
van Steenbergen, H., Band, G. P. H., & Hommel, B. (2010). In the mood for adaptation: How affect regulates conflict-driven control. Psychological Science, 21, 1629–1634. doi:10.1177/0956797610385951
van Steenbergen, H., Band, G. P. H., Hommel, B., Rombouts, S. A., & Nieuwenhuis, S. (2014). Hedonic hotspots regulate cingulate-driven adaptation to cognitive demands. Cerebral Cortex. Advance online publication. doi:10.1093/cercor/bht416
van Steenbergen, H., Booij, L., Band, G. P. H., Hommel, B., & van der Does, A. J. (2012). Affective regulation of cognitive-control adjustments in remitted depressive patients after acute tryptophan depletion. Cognitive, Affective, & Behavioral Neuroscience, 12, 280–286. doi:10.3758/s13415-011-0078-2
Vasterling, J. J., Duke, L. M., Brailey, K., Constans, J. I., Allain, A. N., Jr., & Sutker, P. B. (2002). Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology, 16, 5–14. doi:10.1037/0894-4105.16.1.5
Vythilingam, M., Blair, K. S., McCaffrey, D., Scaramozza, M., Jones, M., Nakic, M., & Blair, R. J. (2007). Biased emotional attention in post-traumatic stress disorder: A help as well as a hindrance? Psychological Medicine, 37, 1445–1455. doi:10.1017/S003329170700092X
Waxer, M., & Morton, J. B. (2011). The development of future-oriented control: An electrophysiological investigation. NeuroImage, 56, 1648–1654. doi:10.1016/j.neuroimage.2011.02.001
Wechsler, D. (1997). Wechsler memory scale–third edition (WMS-III). San Antonio, TX: Psychological Corp.
Wingenfeld, K., Spitzer, C., Mensebach, C., Grabe, H. J., Hill, A., Gast, U., & Driessen, M. (2010). The German version of the Childhood Trauma Questionnaire (CTQ): Preliminary psychometric properties. Psychotherapie, Psychosomatik, Medizinische Psychologie, 60, e13. doi:10.1055/s-0030-1247564
Wittchen, H.-U., & Pfister, H. (1997). DIA-X-Interviews: Manual für Screening-Verfahrenund Interview; Interviewheft Längsschnittuntersuchung (DIA-X-Lifetime); Ergänzungsheft (DIA-X-Lifetime); Interviewheft Querschnittuntersuchung (DIA-X-12Monate); Ergänzungsheft (DIA-X-12 Monate); PC-Programm zur Durchführung des Interviews (Längs- und Querschnittuntersuchung); Auswertungsprogramm. Frankfurt, Germany: Swets & Zeitlinger.
Wu, J., Ge, Y., Shi, Z., Duan, X., Wang, L., Sun, X., & Zhang, K. (2010). Response inhibition in adolescent earthquake survivors with and without posttraumatic stress disorder: A combined behavioral and ERP study. Neuroscience Letters, 486, 117–121. doi:10.1016/j.neulet.2010.07.040
Author note
This work was supported by the German Research Foundation (DFG; Grant Nos. STA 1213/5-1 to T.S. and J.H., and SFB 940/1 Project No. B5 to F.P. and C.K.). The authors are grateful to Stefan Uhmann, who vitally assisted patient recruitment, and to Elisabeth Cohors-Fresenborg, Anna-Katharina Richter, and Juliane Kant for their great help in data collection. The authors also thank the staff of the outpatient unit and the members of the endocrinology laboratory at the Department of Psychology at Technische Universität Dresden. J.H. received speaking honoraria from Astra-Zeneca. All other authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Steudte-Schmiedgen, S., Stalder, T., Kirschbaum, C. et al. Trauma exposure is associated with increased context-dependent adjustments of cognitive control in patients with posttraumatic stress disorder and healthy controls. Cogn Affect Behav Neurosci 14, 1310–1319 (2014). https://doi.org/10.3758/s13415-014-0299-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-014-0299-2