Abstract
Children and older adults often show less favorable reward-based learning and decision making, relative to younger adults. It is unknown, however, whether reward-based processes that influence relatively early perceptual and attentional processes show similar lifespan differences. In this study, we investigated whether stimulus–reward associations affect selective visual attention differently across the human lifespan. Children, adolescents, younger adults, and older adults performed a visual search task in which the target colors were associated with either high or low monetary rewards. We discovered that high reward value speeded up response times across all four age groups, indicating that reward modulates attentional selection across the lifespan. This speed-up in response time was largest in younger adults, relative to the other three age groups. Furthermore, only younger adults benefited from high reward value in increasing response consistency (i.e., reduction of trial-by-trial reaction time variability). Our findings suggest that reward-based modulations of relatively early and implicit perceptual and attentional processes are operative across the lifespan, and the effects appear to be greater in adulthood. The age-specific effect of reward on reducing intraindividual response variability in younger adults likely reflects mechanisms underlying the development and aging of reward processing, such as lifespan age differences in the efficacy of dopaminergic modulation. Overall, the present results indicate that reward shapes visual perception across different age groups by biasing attention to motivationally salient events.
Similar content being viewed by others
Introduction
Monitoring and evaluating outcomes of actions (e.g., reward or benefit of an action or payoffs of a choice) is essential for adaptive behavior. A large body of work has demonstrated that organisms are sensitive to reward distributions in the environment (e.g., Montague, Dayan, Person, & Sejnowski, 1995; Shafir, Wiegmann, Smith, & Real, 1999) and that reward guides learning, influences decision making, and directs action selection (Kurniawan, Guitart-Masip, & Dolan, 2011; Montague, Hyman, & Cohen, 2004; Niv & Schoenbaum, 2008). However, only recently has research started to address the question of whether reward already influences earlier and, presumably, more “automatic” perceptual and attentional orienting or alerting processes. Emerging evidence from this line of work indicates that reward can guide attention relatively early during visual processing, thereby affecting the perception and selection of visual stimuli in the environment (Anderson, Laurent, & Yantis, 2011; Della Libera & Chelazzi, 2006; Hickey, Chelazzi, & Theeuwes, 2010; Kiss, Driver, & Eimer, 2009; Raymond & O’Brien, 2009; Serences, 2008). These interactions between reward and early attentional processes may affect orienting behavior but may also play a critical role during foraging and economic decision making (Robertson, Watamura, & Wilbourn, 2013; Towal, Mormann, & Koch, 2013; Wolfe, 2013).
The mesolimbic and frontal-striatal dopaminergic pathways are critically involved in reward processing (Cools, 2011; D’Ardenne, McClure, Nystrom, & Cohen, 2008; Schultz, Dayan, & Montague, 1997; Tobler, Fiorillo, & Schultz, 2005). The efficacy of dopaminergic, as well as other catecholaminergic, neuromodulation undergoes maturation and senescence in the course of development across the lifespan. There is ample evidence for aging-related declines of pre- and postsynaptic markers of the dopamine system, which have negative consequences on a variety of cognitive functions in older adults (for reviews, see Bäckman Nyberg, Lindenberger, Li, & Farde, 2006; Li, Lindenberger, & Bäckman, 2010; for a neurocomputational integration, see Li, Lindenberger, & Sikström, 2001). In children, there is evidence that adultlike subcortical dopamine levels are reached during middle childhood, whereas dopamine receptor function shows a more gradual developmental trajectory (Baddeley & Diamond, 1996; Diamond, 2007; Haycock et al., 2003). Given these lifespan age differences in dopaminergic neuromodulation, it has been suggested that the sensitivity to reward information and the ability to use reward information for learning, outcome monitoring, or decision making may change across the lifespan (Eppinger, Hämmerer, & Li, 2011; Hämmerer & Eppinger, 2012; Mohr, Li, & Heekeren, 2010). Indeed, children and adolescents often react more strongly to reward information than do younger adults (Crone, Jennings, & Van der Molen, 2004; Eppinger, Mock, & Kray, 2009; van Leijenhorst, Crone, & Bunge, 2006), and older adults are often found to be less sensitive in processing reward information (Eppinger, Kray, Mock, & Mecklinger, 2008; Eppinger, Nystrom, & Cohen, 2012; Hämmerer, Li, Müller, & Lindenberger, 2011; Samanez-Larkin et al., 2011).
In addition to the age-related differences in reward processing, the ability to selectively attend to relevant information changes substantially across the lifespan. During maturation, children and adolescents show deficits in selective attention (Hommel, Li, & Li, 2004; Passow et al., 2013; Rueda et al., 2004; Waszak, Li, & Hommel, 2010). In the course of healthy aging, the ability to selectively focus on task-relevant information declines (Hasher & Zacks, 1988; Passow et al., 2012; Passow et al., 2014; Störmer, Li, Heekeren, & Lindenberger, 2013; see Guerreiro, Murphy, & Van Gerven, 2010, for a review). These changes in selective attention and reward processing across the lifespan give rise to important questions that are yet unanswered: Can reward value modulate attentional selection across the lifespan? What are the cognitive and motivational mechanisms that mediate the effects of reward value on selective attention across different age groups? To what extent may the effect of reward modulation of selective attention differ between age groups?
In the study reported here, we investigated how reward value affects visual attention across the lifespan. Children, adolescents, younger adults, and older adults performed a visual search task in which the target colors were associated with either high or low reward values. Importantly, the feature carrying the reward information (i.e., colors of the target stimuli) was manipulated orthogonally to the goal of the visual search task and was, therefore, task irrelevant. We employed three measures to examine whether reward value affects stimulus selection. First, we computed median response times (RTs) for high- and low-reward target stimuli, separately for the four age groups. Shorter RTs for high-value targets would indicate that high reward value speeds up attentional selection, rendering search more efficient (cf. Duncan & Humphreys, 1989; Wolfe, 1994). Second, for each participant, we computed a reward RT benefit score by taking the differences between RTs from high and low reward value trials and then dividing the differences by individual mean RT of the low-reward trials. By taking this ratio, we control for age and individual differences in baseline RT, and thus this reward score allows us to examine the reduction of RT due to high reward that is proportional for a given individual’s baseline RT. Third, as an index of response consistency, we computed within-person trial-by-trial RT variability for high- and low-reward target stimuli. High within-person performance variability has been associated with deficits in dopaminergic neuromodulation (MacDonald, Li, & Bäckman, 2009a; MacDonald et al., 2012; Papenberg et al., 2011; Papenberg et al., 2013; for neurocomputational simulations, see Li et al., 2001; Li, von Oertzen, & Lindenberger, 2006; ). Reduced RT variability for high-value targets could indicate that higher reward value enhances representational distinctiveness (see, e.g., the simulated effects in Eppinger et al.,2011) and, consequently, reduces decision noise, as reflected in RT variability. Furthermore, in a second task, participants were asked to maintain geometrical shapes in working memory while concurrently performing the reward-based visual search task. This task was included to explore how increased working memory load influences the formation of stimulus–reward associations across the different age groups. Lastly, to explore whether the participants were aware of the associations between reward value and target color, we asked participants about their subjective color preferences in a postexperiment rating.
To foreshadow our results, we found that high reward value significantly speeded up visual search times in all four age groups. Planned follow-up contrast analysis revealed that younger adults showed more sensitivity to reward value, as compared with all other age groups. Furthermore, there were clear age differences in response consistency for low- and high-reward target stimuli: Only younger adults benefited from high reward value to respond more consistently. The additional working memory task eliminated any reward-based benefits in the search task across all age groups.
Method
Participants
A total of 109 healthy right-handed volunteers participated in the study. All participants gave written informed consent and received monetary compensation for the study (minimum of 17 Euros). The ethics committee of the Max Planck Institute for Human Development approved the study. Close visual acuity was assessed prior to the experiment by having participants read digits at regular reading distance (~30 cm) binocularly; color vision was assessed using a set of six color panels (Velhagen & Broschman, 2005). All measurements were taken with optimal ocular correction. Data from 3 children and 5 older adults had to be excluded because of low color vision and/or low visual acuity. Thus, the final sample contained 26 children (8–11 years of age; mean age, 10 years; 14 female), 25 adolescents (14–16 years of age; mean age, 15 years; 14 female), 25 younger adults (20–29 years of age; mean age, 25 years; 14 female), and 25 older adults (62–75 years of age; mean age, 67 years; 14 female). These participants were all within the normal range of close vision (v > 0.5; cf. Cline, Hofstetter, & Griffin, 1997) and could report at least five out of six color panels correctly. Participants were assessed on psychometric tests of semantic knowledge (Lehrl, 1977) and perceptual speed (Wechsler, 1958). Consistent with previous findings (cf. Li et al., 2004), older adults attained lower scores in perceptual speed and higher scores in semantic knowledge than younger adults and adolescentsFootnote 1 (Table 1).
Stimuli and procedure
The testing session took place in age-homogeneous groups of 3–5 individuals and took approximately 2 h. Participants first completed computerized tests of perceptual speed (identical pictures) and semantic knowledge (spot-a-word). Then, participants performed task 1. After a short break of about 10–15 min, participants performed task 2. Participants were paid contingent upon performance in the two experiments: They received a fixed amount of three Euros as a start value and earned additional compensatory money during the experiments (1 or 5 cents for each correct trial). This payment protocol resulted in a final amount of 17.00–20.28 Euros. At the end of the session, participants completed a paper-and-pencil biographical questionnaire. Finally, participants were asked about their color preferences using a computer-based rating task.
Stimuli
Stimuli were presented on a 19-in. computer display (1,024 × 768, 85 Hz) using the Psychophysics Toolbox (Brainard, 1997) extension for MATLAB (The MathWorks). Each display consisted of eight colored letter stimuli (see Fig. 1a). The stimuli were presented on a gray background (10.5 cd/m2) within 9.5° × 15° rectangular regions that were centered to the left and right of the vertical midline. The stimuli were presented at random locations across the visual field, with the constraint that four stimuli appeared in each visual hemifield, with a minimum distance of 1.5° (border to border). Each display contained a “T” as a target letter (1.2° length, 0.4° width) that was tilted to the left or right side. Each display also contained seven distractor letters (the letter “L,” 1.2° length, 0.4° width). On half of the trials, the target letter “T” was presented in the left visual field, and on the remaining half of the trials, the target was presented in the right visual field. Similarly, the target was tilted to the left side on half of the trials and tilted to the right side on the remaining half of the trials. For each experimental block, nine new colors were randomly chosen from the CIElab color space with a distance of 40° on the color wheel. In each experimental block, two colors on the opposite sides of the color distribution were assigned to the target letter T (e.g., green and red; see Fig. 1a), and the remaining seven colors were randomly assigned to the distractor letters. That is, each of the distractors was presented in a distinct color, and the color of the target letter varied between the two chosen target colors from trial to trial in a given block (e.g., green and red; see Fig. 1a). The two target colors were distinctly associated with high and low reward values for correct responses (e.g., red with low reward and green with high reward; see the Procedure section for details). The association between reward value and target color was kept constant throughout each experimental block, but a unique set of colors was used for every experimental block. Importantly, a new set of nine colors was chosen on every block, such that high and low rewards were associated with unique colors that did not repeat across the experimental blocks. Altogether, six blocks were presented; thus, there were overall six high-reward colors and six low-reward colors across the entire first task. The target color varied randomly from trial to trial. The same set of colors was used for all participants; however, high and low reward value colors were counterbalanced between participants of each age group.
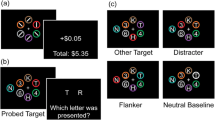
Example trial sequences. a Example trial structure for the reward-based visual search task (task 1) for a high (left) and low (right) reward value trial. Correct responses were rewarded with 5 cents or 1 cent, respectively. The reward values were linked to the target colors, but participants did not know the color of the target prior to the search display. The target colors remained the same within an experimental block but varied across blocks (altogether, six blocks were presented). b Example trial structure for the second task that included a memory task in addition to the reward-based visual search task (task 2). Participants were asked to remember the shapes that were presented along the vertical meridian for two search trials and then to indicate whether a test display matched the shapes presented in the memory display (shown here is a no-match case with set size 3). c Three example displays of the preference rating at the end of the experimental session. Participants were instructed to choose which color they liked better by pressing a left or right response button (the colors were target colors that were either high or low values during the six visual search blocks)
Procedure
Task 1: Reward-based visual search
Participants were seated approximately 70 cm in front of the computer display. Each trial started with the presentation of a central fixation cross (black; 0.45° × 0.45°) for 1 s, which was followed by a search array. Participants had to find the T in each display and report its orientation (left or right) with a buttonpress (left and right control keys). The search array disappeared when a response was registered or after a preset search interval was reached. Subsequently, a feedback screen was presented for 1 s. After a correct response, the feedback screen informed the participant of the monetary reward and displayed either “+1 cent” or “+5 cents” (see example trial structure in Fig. 1a). The reward value depended on the target color on that particular trial. Within each block, one target color was associated with a high reward value (+5 cents), and the other target color was associated with a low reward value (+1 cent). Participants were not informed about the association between target color and reward value. If the response was incorrect, no feedback was provided, and a plain gray screen was presented for 1 s. If the participant failed to respond in the preset time interval, the words “too slow” appeared on the screen, indicating a time-out trial. The preset search interval was implemented to enforce speeded responses. For each participant, it started with a fixed time of 1.5 s. To adjust for age differences in processing speed (see Table 1), the search interval was adjusted individually on the basis of the cumulative percentage of time-out trials (cf. Eppinger et al., 2008; Eppinger et al., 2009). When the cumulative percentage of time-outs exceeded 2%, search time was added in steps of 150 ms for every additional 2% of time-outs. Likewise, when time-outs decreased, search time was reduced in 150-ms steps. The intertrial interval was 1–1.5 s (jittered). Every 16 trials, a screen appeared that informed participants about their earnings (i.e., “You earned XX Euros out of possible XX Euros”). All trial types were randomly intermixed, but the same randomized sequence was used for each age group. The first task consisted of six blocks of 64 trials each. Prior to the task, participants completed 8 practice trials with no monetary reward.
Task 2: Reward-based visual search with memory load
The second task followed the same basic design as task 1. In addition to the visual search task, participants were asked to perform a short-term memory task. Every second search trial, a memory display that contained a set of shape outlines appeared for 4 s (Fig. 1b). The shapes were presented along the vertical meridian. On one third of the trials, three shapes were presented (set size 3); on another third of the trials, four shapes were presented (set size 4); and on the remaining third of the trials, five shapes were presented (set size 5). The shapes included the outer contours (black) of a circle (~1.5° × 1.5°), a square (1.5° × 1.5°), two rectangles oriented vertically or horizontally (0.9° × 2.3° or 2.3° × 0.9°), and two ovals oriented vertically or horizontally (~0.9° × 2.3° or ~2.3° × 0.9°). Shapes were chosen randomly on each trial, but no shape appeared twice in each memory display. Participants were instructed to remember the shapes and their locations. After completing two search trials, a memory test display was presented. On half of the trials, the test display matched the memory array presented before; on the remaining half of the trials, the test array differed from the memory array. The items on the test display could differ from the items on the memory display in their identity, location, or both. Participants had to indicate whether the test array matched the memory array or not by pressing one of two response buttons. To ensure the same maintenance period for each individual, the intertrial interval of the search task was adjusted individually, such that each trial lasted 3.3 s for each participant, regardless of the RTs on the search trials. Thus, the overall maintenance period lasted for about 6.6 s. All trial types were randomly intermixed, but the same randomized sequence was used for each age group. Task 2 consisted of three blocks of 64 search trials and 32 memory trials each.
Color preference rating
Participants’ color preferences were acquired in the following way. High and low reward value colors were paired randomly and presented simultaneously, one color on the left and the other color on the right side of a computer screen (each colored square: 2° × 2°; see Fig. 1c). Each color was presented twice, each time in a new randomly chosen pair. Side of presentation (left/right) was counterbalanced. Participants were instructed to press the response button that corresponded to the color they liked best (i.e., left/right control keys on a keyboard). It was emphasized that there were no correct responses.
Data analysis
Task 1
To examine how reward value influences search behavior, median RTs from correct trials and RT variability were computed separately for each reward value condition (high vs. low) and age group (children, adolescents, younger adults, older adults). Furthermore, a reward RT benefit score was computed by taking the differences between high and low reward value trials and then dividing the differences by individual mean RT on low-reward trials {[RT(low reward) − RT(high reward)]/RT(low reward)}. To obtain a variability measure independent of mean RT differences between the age groups, we computed the coefficient of variation (CV) as an index of processing variability (cf. Papenberg et al., 2011). The CV is a normalized measure of variability. It takes into account given RT baseline differences and, thus, informs about the relative differences in variations given a certain baseline RT in each age group. The CV is defined as the ratio of the standard deviation of RT to the mean RT. Dependent measures were analyzed using a repeated measures analysis of variance (ANOVA) with age group as a between-subjects factor and reward value as a within-subjects factor. When reliable age effects were ascertained, follow-up analyses were performed separately for each age group. Greenhouse–Geisser corrected p-values were applied when appropriate.
Task 2
Short-term memory accuracy (percent correct) was computed separately for each age group and set size condition and was analyzed by means of an ANOVA with age group as a between-subjects factor and memory load as a within-subjects factor. As for task 1, search median RT and search RT variability were analyzed using repeated measures ANOVAs with age group as a between-subjects factor and reward value and memory load as within-subjects factors.
Results
Task 1. Reward-based visual search
As was expected, participants’ accuracy was, overall, high in the search task (between 84% and 95% correct; see Supplementary Material for details). Participants responded faster on trials on which the target color was associated with high reward value, as compared with trials on which the target color was associated with low reward value, F(3, 97) = 53.01, p < .0001, η 2 = .20 (Fig. 2a). Furthermore, we observed a main effect of age group on RT, F(3, 97) = 49.46, p < .0001, η 2 = .58. As was expected, older adults responded slowest, followed by children, adolescents, and younger adults. All pairwise comparisons were significant (all ps < .006). An age group × reward value interaction indicated age differences in the reward value effect, F(3, 97) = 2.81, p = .044, η 2 = .02. Follow-up analyses revealed that each age group showed a value effect on RT but that the magnitude of the effect differed numerically between the age groups [children, 27 ms (±3.72), t(25) = 3.12, p = .005, η 2 = .28; adolescents, 18.7 ms (±2.6), t(24) = 3.68, p = .001. η 2 = .36; younger adults, 48 ms (±4.7), t(24) = 5.78, p < .0001, η 2 = .58; older adults, 58.6 ms (±9.7), t(25) = 3.43, p = .002, η 2 = .33]. These differences between conditions across the age groups point to the largest effect size in younger adults (η 2 = .58). To follow up the age group × reward value interaction in more detail and to control for age and individual differences in baseline RT, a reward RT benefit score was computed separately for each individual by dividing the RT difference between the high and low reward value by the individual mean RT for low reward value. Thus, this reward RT benefit score reflects the reduction of RT due to high reward that is proportional for a given participant’s baseline RT. In light of the numerical differences between the age groups, we conducted planned contrast analyses to test the assumption that younger adults showed the largest effects of reward value on RT. We first compared the RT benefit score separately between children, adolescents, and older adults and found no differences (all ps > .22). After establishing this, we contrasted the group of younger adults against all other three groups ([3, −1, −1, −1]) and found that the magnitude of the RT benefit scores was larger for younger adults, relative to all other groups, F(3, 97) = 4.84, p = .03). Thus, younger adults were more sensitive to reward value than were children, adolescents, and older adults.
Finally, we also plotted at what rate the effect of reward evolved throughout the experiment by computing the reward value RT benefit across trials in each block. Sixteen subsequent trials of each block were combined across all six experimental blocks, revealing four separate bins. As is shown in Fig. 3, the temporal trajectories of the RT value benefit evolved gradually over time across all age groups. Statistical analysis confirmed a main effect of bin, F(3, 9) = 34.50, p < .001, η 2 = .16, but the age group × bin number interaction was not significant (p > .15). Visual inspection of the trajectories suggests that younger and older adults show a similarly steep increase of the reward effects initially, until older adults reach an asymptote at about 30 trials into the block; in contrast, younger adults’ RT benefit further increases later in the block. Children and adolescents appear to show a less steep initial increase of RT benefits, as compared with younger and older adults. On the basis of these observations, we selectively compared the initial mean slopes (bin 1 with bin 2) between children versus adolescents and younger versus older adults, showing no differences between these pairwise comparisons (all ps > .26). Then we collapsed across children and adolescents and contrasted their mean slopes against the mean slopes of younger and older adults. This analysis revealed that the initial mean slopes (bin 1 to bin 2) of younger and older adults significantly deviated from the mean slopes of children and adolescents, F(1, 99) = 4.59, p = .035, η 2 = .18.
Temporal trajectories of the reward effect [(low reward value − high reward value)/low reward value] on response time in task 1. Sixteen subsequent trials of each block are combined across all six blocks. The temporal trajectories show a gradual increase of the reward effect over time for all age groups
To test whether reward value affects within-person variability, we examined the individual RT variability (CV) separately for high and low reward value trials. The analysis revealed a main effect of age group, F(3, 97)=10.65, p < .0001, η 2 = .19, a main effect of reward value, F(3, 97) = 9.56, p = .003, η 2 = .23, and an age group × reward value interaction, F(3, 97) = 2.96, p = .031, η 2 = .11. As can be seen in Fig 2b, only younger adults had lower response variability for high-value trials, relative to low-value trials [younger adults, 4.2% (±0.25), t(24) = 3.59, p = .002, η 2 = .35], whereas all other age groups did not [children, 0.96% (±0.28), t(25) = 1.71, p = .10 η 2 = .10; adolescents, −0.2% (±0.17), t(24) = 0.528, p = .602 η 2 = .01; older adults, 0.17% (±0.19), t(24) = 0.46, p = .646, η 2 = .01]. In sum, the results indicate that higher reward value speeded search times in all age groups; this effect was most pronounced in younger adults; furthermore, reward value reduced within-person variability in younger adults only.
Task 2. Reward-based visual search with memory load
Short-term memory task
Older adults and children had lower accuracy rates, relative to younger adults and adolescents (Table 2). We observed a main effect of age group, F(3, 97) = 19.61, p < .0001, η 2 = .22, and a main effect of working memory load, F(3, 97) = 69.26, p < .0001, η 2 = .17, but no age group × load interaction, F(3, 97) = 0.55, p = .73, η 2 = .004. To examine at which level the effect of working memory emerged, pairwise comparisons were conducted for the different set size conditions. Working memory accuracy dropped significantly from set size 3 to set size 4, F(1, 97) = 174.18, p = .0001, η 2 = .22, and from set size 3 to set size 5, F(1, 97) = 92.50, p = .0001, η 2 = .19, but there was no difference between accuracy rates from set size 4 to set size 5 (p = .69). No interactions with age group were observed in any of these pairwise comparisons (all ps > .51). Thus, across all age groups, working memory performance decreased similarly from set size 3 to set sizes 4 and 5.
Visual search task
Median RT and the CV were examined with an ANOVA with age group as a between-subjects factor and memory load (high [collapsed across set sizes 4 and 5], low (set size 3]) and reward value (high, low) as within-subjects factors. There was a main effect of age group [median RT, F(3, 97) = 48.15, p < .0001, η 2 = .60; CV, F(3, 97) = 4.73, p = .004, η 2 = .12], similar to task 1. All pairwise comparisons were significant (all ps < .004). As can be seen in Fig. 4, no differences between high and low reward value trials were found [median RT, F(1, 99) = 2.27, p = .135; CV, F(1, 99) = 2,99, p = .09]. Also, no memory load × reward value interaction was present [median RT × memory load, F(1, 99) = 0.43, p = .51; CV × memory load, F(1, 99) = 0.12 p = .72], and no age group interactions were observed (all ps > .14). Thus, the additional short-term memory task completely eliminated the effect of reward value during visual search.
Postexperimental color preference rating
Color preferences were examined by comparing the frequencies of how often participants chose the high reward value color, relative to the low reward value color. We observed an age group × reward value interaction that reached significance, F(1, 94) = 2.69, p = .051. To further explore age group differences in the ratings, we performed post hoc comparisons for each age group separately and found that younger adults reported the high reward value color as “likable” more often (58.3%), relative to the low reward value color (41.7%), t(23) = 2.07, p = .04, η 2 = .16; none of the other age groups showed this tendency (all other ps > .14).
Discussion
Across four different age groups, we found that stimulus–reward associations can influence stimulus selection in a visual search task. When targets were associated with high reward value, participants responded faster, as compared with when targets were associated with low reward value. This effect appeared to be larger in younger adults than in children, adolescents, and older adults. Interestingly, only younger adults showed lower within-person trial-to-trial response variability when targets were associated with high reward value, as compared with when targets were associated with low reward value. Thus, our findings show that higher reward value accelerated target detection in all four age groups, with the effect being largest in younger adults. Furthermore, higher reward value rendered more consistent behavior in younger adults only.
Our result showing the largest effects of reward value in younger adults is in good agreement with previous studies on developmental and aging-related differences and impairments in reward-based processes (Chowdhury et al., 2013; Eppinger et al., 2008; Hämmerer et al., 2011; Hämmerer et al., 2013; Nieuwenhuis et al., 2002; Samanez-Larkin, Levens, Perry, Dougherty, & Knutson, 2012; van Duivenvoorde, Zanolie, Rombouts, Raijmakers, & Crone, 2008). However, although we find stronger effects of reward value in younger adults, all three other age groups showed pronounced effects of reward value on response speed. This indicates that reward can affect perceptual and attentional processes across the lifespan and suggests that children, adolescents, and older adults are sensitive to reward value associations in the context of visual search tasks. Different from the present study, most of the learning and decision-making tasks that show strong developmental and age impairments in performance are tasks that involve explicit goal-directed behavior. That is, participants are asked to learn stimulus–response associations based on rewarding or aversive outcomes or to make decisions to maximize reward. In contrast, in the present task, the feature carrying the value information (i.e., color) was orthogonal to the search task and, therefore, task irrelevant. In fact, performance and monetary gain at the end of the experiment was completely independent of whether participants learned the association between color and reward value or not. The fact that participants nevertheless acquired the association between value and color (as reflected in the RT speed-up) suggests that the underlying mechanism operates at relatively early and implicit processing stages. Initial support for this view comes from a postexperiment rating in which we asked participants about their subjective preferences for the colors that were previously associated with high and low reward value. Observers were instructed to pick the color they liked better in a two-alternative forced choice task, in which high and low reward value colors were contrasted against each other. This rating procedure allowed us to explore whether participants’ preferences were shifted toward the high value color—without having to tap into goal-directed, explicit processes. Whereas younger adults showed a tendency to report the high reward value color as more “likable,” none of the other age groups showed preferences for one or the other color. This suggests that most of the participants (at least, children, adolescents, and older adults) were not biased toward high reward value colors, implying that these associations were rather weak and of an implicit nature. Furthermore, the analysis of the temporal trajectories (Fig. 3) showing a slow and gradual development of RT value effects over time further supports the hypothesis that reward magnitude associations were learned gradually and, thus, rather implicitly. Taken together, these data are nicely consistent with other recent results suggesting that reward effects on visual attention emerge involuntarily and independently of task goals. For instance, Anderson et al. (2011) found that the presence of a task-irrelevant item in a search array that was previously associated with high reward value slows down visual search—presumably, by capturing attention automatically (see also Anderson et al., 2012, 2013; Anderson & Yantis, 2013; Krebs, Boehler, Egner, & Woldorff, 2011; Krebs, Boehler, & Woldorff, 2010; Yantis, Anderson, Wampler, & Laurent, in press). More evidence comes from electrophysiological recordings in the posterior parietal cortex of the monkey showing sustained activation for reward-associated stimuli, even when such reward-dependent activation is suboptimal for the task, implying that reward-associated features render attentional priority regardless of behavioral performance (Peck, Jangraw, Suzuki, Efem, & Gottlieb, 2009). Of additional interest, an EEG study in humans suggests that reward effects in visual search arise within the first 100 ms of visual processing, as reflected in amplitude modulations of the P1 component (Hickey, Chelazzi, & Theeuwes, 2010). Taken together, our findings may suggest that relatively early and involuntary attentional mechanisms and their modulation by reward are present across the human lifespan. Such an interpretation would be consistent with several findings in the aging and developmental literature that suggest that lower-level processes such as implicit learning may be less affected by age (Cherry & Stadler, 1995; Dennis, Howard, & Howard, 2006; Howard, Howard, Dennis, LaVine, & Valentino, 2008; Merrill, Conners, Roskos, Klinger, & Klinger, 2013; Thomas & Nelson, 2001).
Another aspect of our study that differs from previous studies is the fact that most studies on goal-directed learning and decision making focus on the direct effects of reward and punishments on behavior, whereas in the present experiment, we were primarily interested in the effects of reward value (low vs. high) on selective attention in different age groups. The finding of the reward-based speed-up in RT across all age groups may hence also be a consequence of the fact that differences in value are less salient than bivalent outcomes that signal approach and avoidance.
The result that high reward value led to more consistent behavior in younger adults indicates that although mean RT and response variability share common variance, intraindividual RT variability provides an index beyond that of mean RT (Li, Hämmerer, Müller, Hommel, & Lindenberger, 2009; Li et al., 2004; Lövdén, Li, Shing, & Lindenberger, 2007; Schmiedek, Lövdén, & Lindenberger, 2009). Indeed, intraindividual RT variability (or lack of response consistency) has been shown to be a more sensitive measure in reflecting age- or pathology-related effects (MacDonald, Nyberg, & Bäckman, 2006). For instance, RT variability has been linked to dopaminergic neuromodulation. This notion comes from computational models (Li et al., 2001; Li et al., 2006) and dopamine receptor imaging studies (MacDonald, Cervenka, Farde, Nyberg, Bäckman, 2009b), which demonstrated relations between suboptimal dopaminergic neuromodulation and higher activation fluctuations (i.e., noise) in neural networks and behavioral performance variability, respectively. Specifically, computationally it has been demonstrated that simulating higher dopamine modulation in younger adults by a larger gain regulation of the signal-to-noise ratio of neural information processing resulted in less random activation variability and less trial-by-trial performance variability (e.g., Li et al., 2001; cf. Servan-Schreiber, Printz, & Cohen, 1990). Empirical evidence lends further support to this effect. A recent finding shows that higher dopamine receptor efficacy is associated with lower trial-by-trial behavioral variability (see MacDonald, Karlsson, Rieckmann, Nyberg, & Bäckman, 2012). Our finding is also in line with recent studies that suggested that age-related impairments in learning and decision making might be due to increased temporal variability in the activity of areas that receive strong dopaminergic input, such as the ventral striatum (Chowdury et al., 2013; Eppinger et al., 2013; Samanez-Larkin et al., 2010). Thus, it seems plausible that more optimal dopaminergic neuromodulation in younger adults would affect the quality of reward-feature templates by making them less noisy, which in turn would render more consistent behavior. This is exactly what we observed for high-value targets in younger adults. Possibly, the more distinct representations also influenced the postexperiment preference ratings, in which only younger adults tended to choose the high reward value color more often. A possible candidate accounting for these age-specific value effects on RT variability are developmental and aging-related differences in the baseline levels of dopaminergic neuromodulation. Estimates from cross-sectional studies suggest that the aging-related dopamine decline in striatal and extrastriatal regions progresses with a rate of about 10% per decade, starting in the third decade of life (e.g., Inoue et al., 2001; Kaasinen et al., 2000; see Bäckman et al., 2006, for a review). With respect to child development, the literature is much scarcer due to reservations in applying invasive methods, such as PET receptor imaging. The available evidence suggests that adultlike subcortical dopamine levels are reached during middle childhood (Haycock et al., 2003; Seeman et al., 1987), whereas dopamine receptor function shows a more gradual developmental trajectory (Baddeley & Diamond, 1996; Diamond, 2007; Rothmond, Weickert, & Webster, 2012). Possibly, these developmental and aging-related differences in dopaminergic neuromodulation underlie the observed differences in value effects on RT variability. This is also consistent with recent findings showing lifespan age differences in trial-by-trial variability of EEG signals in a performance-monitoring task (Papenberg, Hämmerer, Müller, Lindenberger, & Li, 2013).
In contrast to the well-established role of dopamine prediction error signaling for reinforcement learning (for a review, see Niv & Schoenbaum, 2008), the neural mechanisms that underlie the effects of reward on early perceptual and attentional processes are not well understood. EEG and MEG data point to value-related modulations at early visual processing stages (Chelazzi, Perlato, Santandrea, & Della Libera, 2013; Hickey et al. 2010; Tallon-Baudry Meyniel, & Bourgeois-Gironde, 2011), and results from fMRI research show that value signals can modulate activity in the early visual cortex, including V1 (Seitz, Kim, & Watanabe, 2009; Serences, 2008). For example, one study found enhanced activity in higher nonretinotopic visual areas (in addition to the striatum and orbitofrontal cortex) during the presentation of rewarding feedback, which, on the following trial, led to increased activity in early visual areas (V1, V2) that was spatially specific (i.e., contralateral to the target; Weil et al., 2010). This suggests that rewards may initially trigger rather global, nonspecific signals, which subsequently influence activity in the early visual cortex (Weil et al., 2010). These findings are also consistent with findings in monkeys that show value-dependent modulations of neuronal activity in sensory areas as early as V1 (Arsenault, Nelissen, Jarraya, & Vanduffel, 2013; Stanisor, van der Togt, Pennartz, & Roelfsema, 2013). Together, these findings may suggest that mesencephalic dopamine can change the perceptual saliency of a rewarded stimulus by directly changing sensory signals at the level of the primary visual cortex (cf. Berridge & Robinson, 1998).
It seems rather unlikely that reward had such a direct influence on visual processing in the present task. More plausible is that brain areas related to selective attention mediated the effects of reward on visual processing. Hickey et al. (2010) recently showed that the anterior cingulate—an area involved in attentional control processes (Hopfinger, Buonocore, & Mangun, 2000)—plays an important role in guiding attention toward motivationally significant events. One tentative interpretation of these findings could be that reward value triggers involuntary attentional mechanisms that facilitate the early processing of reward-associated features. These effects are then reflected in speeded RT and enhanced responses in early visual processing areas (Hickey et al. 2010; Serences, 2008). Support for the argumentation that the present results do not reflect direct effects of reward on visual processing but are mediated through attention comes from the second task. In this part of the study, participants performed the reward-based visual search task but were instructed to additionally engage in a working memory task to increase demands on executive control functions. Across all four age groups, we found that the secondary task slowed down RT. Most interesting, the RT benefit for high reward value, compared with low reward value, disappeared in all age groups. One way to interpret this effect would be to assume that increased demands on control interfere with reward effects in visual search. This would imply that the attentional mechanism that mediates the reward effects is part of a more general executive control network that is involved in guiding behavior toward motivationally significant events. Increased demands on this network may lead to deficits in building up stimulus–reward associations. This interpretation would be in line with an extensive literature on the role of the medial prefrontal cortex in executive control (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Corbetta & Shulman, 2002).
However, it could also be argued that in the dual-task situation, participants shifted their attentional focus to the secondary task and were thus not able to detect the reward associations. This view would be consistent with the idea that the reward value effects reflect early involuntary attentional mechanisms, rather than deliberate executive control processes. Future studies should address this question more directly by trying to dissociate involuntary shifts of attentional focus due to task demands from limitations in executive control due to the secondary task. Note that the search-memory task was always performed after the reward-based search task without the memory component. Thus, the results of the second task should be interpreted with caution. However, control analyses that examined the effect of reward value separately across experimental blocks revealed that although the reward effect seemed to be strongest during initial blocks, substantial reward effects were still observed during later blocks in task 1, in contrast to the complete elimination of the reward effects in task 2 (even in the first block).Footnote 2 Thus, whereas the order effect may moderate the effect of reward to some extent, the complete elimination of reward effect in task 2 cannot be attributed to order effects alone. Future experiments should intermix both trial types to unequivocally relate the lack of reward value on search RT to the memory component.
Taken together, the present empirical results show that reward value speeds visual search times in children, adolescents, younger adults, and older adults. This effect seems to be most pronounced for younger adults and of similar magnitude for all other age groups. Only younger adults showed more consistent behavior for high reward value trials and rated colors previously associated with high value as more “likable.” These findings lead us to propose that relatively early involuntary and implicit stimulus–reward associations can modulate visual attention across the lifespan. At the same time, these findings seem to be consistent with the hypothesis that lifespan age differences in dopaminergic neuromodulation contribute to age differences in reward processes even at these relatively early perceptual and attentional stages, as reflected in age-related differences in behavioral benefit and variability. Interestingly, we also found that reward value effects disappear when a secondary task is added to the visual search paradigm. We think that this effect may be due to a shift in the attentional focus to the more demanding secondary task.
Notes
Data from two adolescents are missing for the perceptual speed and verbal knowledge tasks.
To test for order effects, the magnitude of the reward value effect (low-minus-high reward value RTs) was computed separately for each experimental block. For task 1, the effects were combined across two subsequent blocks, rendering three different bins. The reward value effects appeared larger at the beginning of task 1 (bin 1, 50 ms; bin 2, 42 ms; bin 3, 26 ms). For task 2, there was no hint of a value effect, not even in the first block (bin 1, 7 ms; bin 2, −5 ms; bin 3, 0.1 ms). Statistical tests revealed that the magnitude of the reward effect in task 2, bin 1, was significantly reduced relative to the reward effect in task 1, bin 3 (p = .03) across all age groups (age group × reward value: p = .91), confirming that the secondary task eliminated the reward value effect.
References
Anderson, B. A., Laurent, P., & Yantis, S. (2013). Reward predictions bias attentional selection. Frontiers in Human Neuroscience, 7, 262. doi:10.3389/fnhum.2013.00262
Anderson, B. A., Laurent, P., & Yantis, S. (2011). Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America, 108(25), 10367–10371. doi:10.1073/pnas.1104047108
Anderson, B. A., Laurent, P. A., & Yantis, S. (2012). Generalization of value-based attentional priority. Visual Cognition, 20(6), 647–658. doi:10.1080/13506285.2012.679711
Anderson, B. A., & Yantis, S. (2013). Persistence of Value-Driven Attentional Capture. Journal of Experimental Psychology-Human Perception and Performance, 39(1), 6–9. doi:10.1037/a0030860
Arsenault, J. T., Nelissen, K., Jarraya, B., & Vanduffel, W. (2013). Dopaminergic reward signals selectively decrease fMRI activity in primate visual cortex. Neuron, 77, 1174–1186.
Bäckman, L., Nyberg, L., Lindenberger, U., Li, S., & Farde, L. (2006). The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neuroscience and Biobehavioral Reviews, 30(6), 791–807. doi:10.1016/j.neubiorev.2006.06.005
Baddeley, A., & Diamond, A. (1996). Evidence for the importance of dopamine for prefrontal cortex functions early in life - Discussion. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 351(1346), 1494.
Berridge, K. C., & Robinson, T. E. (1998). What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews, 28(3), 309–369. doi:10.1016/s0165-0173(98)00019-8
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. doi:10.1037//0033-295x.108.3.624
Brainard, D. H. (1997). The psychophysics toolbox. Spatial vision, 10(4), 433–436.
Chelazzi, L., Perlato, A., Santandrea, E., & Della Libera, C. (2013). Rewards teach visual selective attention. Vision Research, 85, 58–72. doi:10.1016/j.visres.2012.12.005
Cherry, K. E., & Stadler, M. A. (1995). Implicit learning of nonverbal sequence in younger and older adults. Psychology and Aging, 10(3), 379–394. doi:10.1037/0882-7974.10.3.379
Chowdhury, R., Guitart-Masip, M., Lambert, C., Dayan, P., Huys, Q., Duezel, E., & Dolan, R. J. (2013). Dopamine restores reward prediction errors in old age. Nature Neuroscience, 16(5), 648. doi:10.1038/nn.3364
Cline, D., Hofstetter, H. W., & Griffin, J. R. (1997). Dictionary of visual science.(4th ed.): Boston, MA: Butterworth.
Cools, R. (2011). Dopaminergic control of the striatum for high-level cognition. Current Opinion in Neurobiology, 21, 402–407.
Corbetta, M., & Shulman, G. L. (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. doi:10.1038/nrn755
Crone, E., Jennings, J., & Van der Molen, M. (2004). Developmental change in feedback processing as reflected by phasic heart rate changes. Developmental Psycholopgy, 40(6), 1228–1238.
D'Ardenne, K., McClure, S. M., Nystrom, L. E., & Cohen, J. D. (2008). BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science, 319(5867), 1264–1267. doi:10.1126/science.1150605
Della Libera, C., & Chelazzi, L. (2006). Visual Selective Attention and the Effects of Monetary Rewards. Psychological Science, 17(3), 222–227. doi:10.1111/j.1467-9280.2006.01689.x
Dennis, N. A., Howard, J. H., Jr., & Howard, D. V. (2006). Implicit sequence learning without motor sequencing in young and old adults. Experimental Brain Research, 175(1), 153–164. doi:10.1007/s00221-006-0534-3
Diamond, A. (2007). Consequences of variations in genes that affect dopamine in prefrontal cortex. Cerebral Cortex, 17, I161–I170. doi:10.1093/cercor/blini082
Duncan, J., & Humphreys, G. W. (1989). Visual-search and stimulus similarity. Psychological Review, 96(3), 433–458.
Eppinger, B., Hämmerer, D., & Li, S.-C. (2011). Neuromodulation of reward-based learning and decision making in human aging. Annals of the New York Academy of Sciences, 1235, Decision-making over the lifespan, 1-17. doi: 10.1111/j.1749-6632.2011.06230.x
Eppinger, B., Kray, J., Mock, B., & Mecklinger, A. (2008). Better or worse than expected? Aging, learning, and the ERN. Neuropsychologia, 46(2), 521–539. doi:10.1016/j.neuropsychologia.2007.09.001
Eppinger, B., Mock, B., & Kray, J. (2009). Developmental differences in learning and error processing: Evidence from ERPs. Psychophysiology, 46(5), 1043–1053. doi:10.1111/j.1469-8986.2009.00838.x
Eppinger, B., Nystrom, L. E., & Cohen, J. D. (2012). Reduced Sensitivity to Immediate Reward during Decision-Making in Older than Younger Adults. Plos One, 7(5), e36953. doi:10.1371/journal.pone.0036953
Eppinger, B., Schuck, N. W., Nystrom, L. E., & Cohen, J. D. (2013). Reduced striatal responses to reward prediction errors in older compared with younger adults. The Journal of Neuroscience, 33(24), 9905–9912.
Guerreiro, M. J. S., Murphy, D. R., & Van Gerven, P. W. M. (2010). The Role of Sensory Modality in Age-Related Distraction: A Critical Review and a Renewed View. Psychological Bulletin, 136(6), 975–1022. doi:10.1037/a0020731
Hasher, L., & Zacks, R. T. (1988). The psychology of learning and motivation. New York: Academic.
Hämmerer, D., Li, S.-C., Müller, V., & Lindenberger, U. (2011). Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. Journal of Cognitive Neuroscience, 23(3), 1–14.
Hämmerer, D., & Eppinger, B. (2012). Dopaminergic and prefrontal contributions to learning and outcome monitoring during childhood and old age. Developmental Psychology, 48, 862–874.
Hämmerer D, Biele G., Müller, V., Thiele H., Nürnberg P, Heekeren, H. R., Li, S.-C. (2013). Effects of PPP1R1B (DARPP-32) polymorphism on feedback-related brain potentials across the lifespan. Frontiers in Psychology, 4, Article 89.
Haycock, J. W., Becker, L., Ang, L., Furukawa, Y., Hornykiewicz, O., & Kish, S. J. (2003). Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry, 87(3), 574–585. doi:10.1046/j.1471-4159.2003.02017.x
Hickey, C., Chelazzi, L., & Theeuwes, J. (2010). Reward Changes Salience in Human Vision via the Anterior Cingulate. Journal of Neuroscience, 30(33), 11096–11103. doi:10.1523/JNEUROSCI.1026-10.2010
Hommel, B., Li, K. Z. H., & Li, S. C. (2004). Visual search across the life span. Developmental Psychology, 40(4), 545–558. doi:10.1037/0012-1649.40.4.545
Hopfinger, J. B., Buonocore, M. H., & Mangun, G. R. (2000). The neural mechanisms of top down attentional control. Nature Neuroscience, 3(3), 284–291.
Howard, D. V., Howard, J. H., Jr., Dennis, N. A., LaVine, S., & Valentino, K. (2008). Aging and implicit learning of an invariant association. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 63(2), 100–105.
Inoue, M., Suhara, T., Sudo, Y., Okubo, Y., Yasuno, F., Kishimoto, T., & Tanada, S. (2001). Age-related reduction of extrastriatal dopamine D-2 receptor measured by PET. Life Sciences, 69(9), 1079–1084. doi:10.1016/s0024-3205(01)01205-x
Kaasinen, V., Vilkman, H., Hietala, J., Någren, K., Helenius, H., Olsson, H., Farde, L., & Rinne, J. (2000). Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiology of Aging, 21(5), 683–688. doi:10.1016/s0197-4580(00)00149-4
Kiss, M., Driver, J., & Eimer, M. (2009). Reward Priority of Visual Target Singletons Modulates Event-Related Potential Signatures of Attentional Selection. Psychological Science, 20(2), 245–251. doi:10.1111/j.1467-9280.2009.02281.x
Krebs, R. M., Boehler, C. N., & Woldorff, M. G. (2010). The influence of reward associations on conflict processing in the Stroop task. Cognition, 117(3), 341–347. doi:10.1016/j.cognition.2010.08.018
Krebs, R. M., Boehler, C. N., Egner, T., & Woldorff, M. G. (2011). The Neural Underpinnings of How Reward Associations Can Both Guide and Misguide Attention. Journal of Neuroscience, 31(26), 9752–9759. doi:10.1523/jneurosci.0732-11.2011
Kurniawan, I. T., Guitart-Masip, M., & Dolan, R. J. (2011). Dopamine and effort-based decision making. Frontiers in Neuroscience, 5, 81.
Lehrl, S. (1977). Mehrfachwahl-Wortschatz-test B [multiple-choice knowledge test-B (MWT-B)]. Erlangen, Germany: Straube.
Li, S.-C., Hämmerer, D., Müller, V., Hommel, B., & Lindenberger, U. (2009). Lifespan development of stimulus-response conflict cost: similarities and differences between maturation and senescence. Psychological Research, 73, 777–785.
Li, S.-C., Lindenberger, U., & Sikström, S. (2001). Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences, 5(11), 479–486. doi:10.1016/s1364-6613(00)01769-1
Li, S.-C., Lindenberger, U., Hommel, B., Aschersleben, G., Prinz, W., & Baltes, P. B. (2004). Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science, 15(3), 155–163.
Li, S.-C., Lindenberger, U., & Bäckman, L. (2010). Dopaminergic modulation of cognition across the lifespan. Neuroscience and Biobehavioral Reviews, 34, 625–630.
Li, S.-C., von Oertzen, T., & Lindenberger, U. (2006). A neurocomputational model of stochastic resonance and aging. Neurocomputing, 69, 1553–3560.
Lövdén, M., Li, S.-C., Shing, Y. L., & Lindenberger, U. (2007). Within-person trial-to-trial variability precedes and predicts cognitive decline in old and very old age: Longitudinal data from the Berlin Aging Study. Neuropsychologia, 45(12), 2827–2838. doi:10.1016/j.neuropsychologia.2007.05.005
MacDonald, S. W. S., Nyberg, L., & Bäckman, L. (2006). Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences, 29(8), 474–480. doi:10.1016/j.tins.2006.06.011
MacDonald, S. W. S., Li, S., & Bäckman, L. (2009a). Neural underpinnings of within person variability in cognitive functioning and aging. Gerontologist, 49, 388.
MacDonald, S. W. S., Cervenka, S., Farde, L., Nyberg, L., & Bäckman, L. (2009b). Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia, 47(11), 2299–2304. doi:10.1016/j.neuropsychologia.2009.01.016
MacDonald, S. W. S., Karlsson, S., Rieckmann, A., Nyberg, L., & Bäckman, L. (2012). Aging-Related Increases in Behavioral Variability: Relations to Losses of Dopamine D1 Receptors. The Journal of Neuroscience, 32(24), 8186–8191. doi:10.1523/JNEUROSCI.5474-11.2012
Merrill, E. C., Conners, F. A., Roskos, B., Klinger, M. R., & Klinger, L. G. (2013). Contextual Cueing Effects Across the Lifespan. Journal of Genetic Psychology, 174(4), 387–402. doi:10.1080/00221325.2012.694919
Montague, P. R., Dayan, P., Person, C., & Sejnowski, T. J. (1995). Bee foraging in uncertain environments using predictive hebbian learning. Nature, 377, 725–728.
Montague, P. R., Hyman, S. E., & Cohen, J. D. (2004). Computational roles for dopamine in behavioural control. Nature, 431(7010), 760–767.
Mohr, P. N. C., Li, S.-C., & Heekeren, H. (2010). Neuroeconomics and Aging: Neuromodulation of economic decision making in old age. Neuroscience and Biobehavioral Reviews, 34, 678–688.
Nieuwenhuis, S., Ridderinkhof, K. R., Talsma, D., Coles, M. G. H., Holroyd, C. B., Kok, A., & Van der Molen, M. W. (2002). A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cognitive, Affective, & Behavioral Neuroscience, 2(1), 19–36.
Niv, Y., & Schoenbaum, G. (2008). Dialogues on prediction errors. Trends in Cognitive Sciences, 12(7), 265–272. doi:10.1016/j.tics.2008.03.006
Papenberg, G., Bäckman, L., Chicherio, C., Nagel, I., Heekeren, H., Lindenberger, U., & Li, S. (2011). Higher intraindividual variability is associated with more forgetting and dedifferentiated memory functions in old age. Neuropsychologia, 49(7), 1879–1888. doi:10.1016/j.neuropsychologia.2011.03.013
Papenberg, G., Hämmerer, D., Müller, V., Lindenberger, U., & Li, S.-C. (2013). Low theta inter-trial phase coherence during performance monitoring is related to higher reaction time variability: A lifespan study. NeuroImage, 83, 912–920. doi:10.1016/j.neuroimage.2013.07.032
Passow, S., Westerhausen, R., Wartenburger, I., Hugdahl, K., Heekeren, H., Lindenberger, U., & Li, S. (2012). Human aging compromises attentional control of auditory perception. Psychology and Aging, 27(1), 99–105. doi:10.1037/a0025667
Passow, S., Westerhausen, R., Hugdahl, K., Wartenburger, I., Heekeren, H. R., Lindenberger, U., & Li, S.-C. (2013). Development of attentional control of verba auditory perception from middle to late childhood: Comparisons to healthy aging. Developmental Psychology, 49, 1982–1993.
Passow, S., Westerhausen, R., Hugdahl, K., Wartenburger, I., Heekeren, H. R., Lindenberger, U., & Li, S.-C. (2014). Electrophysiological correlates of adult age differences in attentional control of auditory processing. Cerebral Cortex, 24, 249–260.
Peck, C. J., Jangraw, D. C., Suzuki, M., Efem, R., & Gottlieb, J. (2009). Reward Modulates Attention Independently of Action Value in Posterior Parietal Cortex. Journal of Neuroscience, 29(36), 11182–11191. doi:10.1523/jneurosci.192909.2009
Raymond, J. E., & O'Brien, J. L. (2009). Selective Visual Attention and Motivation: The Consequences of Value Learning in an Attentional Blink Task. Psychological Science, 20(8), 981–988. doi:10.1111/j.1467-9280.2009.02391.x
Robertson, S. S., Watamura, S. E., & Wilbourn, M. P. (2013). Attentional dynamics of infant visual foraging. Proceedings of the National Academy of Sciences, 109, 11460–11464.
Rothmond, D. A., Weickert, C. S., & Webster, M. J. (2012). Developmental changes in human dopamine neurotransmission: cortical receptors and terminators. BMC Neuroscience, 13. doi: 10.1186/1471-2202-13-18
Rueda, M., Fan, J., McCandliss, B., Halparin, J., Gruber, D., Lercari, L., & Posner, M. (2004). Development of attentional networks in childhood. Neuropsychologia, 42(8), 1029–1040. doi:10.1016/j.neuropsychologia.2003.12.012
Samanez-Larkin, G. R., Kuhnen, C. M., Yoo, D. J., & Knutson, B. (2010). Variability in nucleus accumbens activity mediates age-related suboptimal financial risk taking. The Journal of Neuroscience, 30(4), 1426–1434.
Samanez-Larkin, G. R., Mata, R., Radu, P. T., Ballard, I. C., Carstensen, L., & McClure, S. M. (2011). Age differences in striatal delay sensitivity during the intertemporal choice in healthy adults. Frontiers in Neuroscience, 5, 126.
Samanez-Larkin, G. R., Levens, S. M., Perry, L. M., Dougherty, R. F., & Knutson, B. (2012). Frontostriatal White Matter Integrity Mediates Adult Age Differences in Probabilistic Reward Learning. Journal of Neuroscience, 32(15), 5333–5337. doi:10.1523/jneurosci.5756-11.2012
Shafir, S., Wiegmann, D. D., Smith, B. H., & Real, L. A. (1999). Risk-sensitive foraging: Choice behavior of honeybees in response to variability in volume of reward. Animal Behavior, 57, 1055–1061.
Schmiedek, F., Lovden, M., & Lindenberger, U. (2009). On the Relation of Mean Reaction Time and Intraindividual Reaction Time Variability. Psychology and Aging, 24(4), 841–857. doi:10.1037/a0017799
Schultz, W., Dayan, P., & Montague, R. (1997). A neural substrate of prediction and reward. Science, 275, 1593–1599.
Seeman, P., Bzowej, N. H., Guan, H. C., Bergeron, C., Becker, L. E., Reynolds, G. P., & Tourtellotte, W. W. (1987). Human-brain dopamine-receptors in children and aging adults. Synapse, 1(5), 399–404. doi:10.1002/syn.890010503
Seitz, A. R., Kim, D., & Watanabe, T. (2009). Rewards evoke learning of unconsciously processed visual stimuli in adult humans. Neuron, 61, 700–707.
Serences, J. (2008). Value-Based Modulations in Human Visual Cortex. Neuron, 60(6), 1169–1181.
Servan-Schreiber, D., Printz, H., & Cohen, J. D. (1990). A network model of catecholamine effects: Gain, signal-to-noise ratio, and behavior. Science, 249, 892–895.
Stanisor, L., van der Togt, C., Pennartz, C. M. A., & Roelfsema, P. R. (2013). A unified selection signal for attention and reward in primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 110(22), 9136–9141. doi:10.1073/pnas.1300117110
Störmer, V. S., Li, S.-C., Heekeren, H. R., & Lindenberger, U. (2013). Normal aging delays and compromises early multifocal visual attention during object tracking. Journal of Cognitive Neuroscience, 25(2), 188–202.
Tallon-Baudry, C., Meyniel, F., & Bourgeois-Gironde, S. (2011). Fast and automatic activation of an abstract representation of money in the human ventral visual pathway. PloS One, 6, e28229.
Thomas, K. M., & Nelson, C. A. (2001). Serial reaction time learning in preschool- and school-age children. Journal of Experimental Child Psychology, 79(4), 364–387. doi:10.1006/jecp.2000.2613
Tobler, P. N., Fiorillo, C. D., & Schultz, W. (2005). Adaptive coding of reward value by dopamine neurons. Science, 307, 1642–1645.
Towal, R. B., Mormann, M., & Koch, C. (2013). Simultaneous modeling of visual saliency and value computation improves predictions of economic choice. Proceedings of the National Academy of Sciences of the United States of America, 110(40), E3858–E3867.
van Duijvenvoorde, A. C. K., Zanolie, K., Rombouts, S. A. R. B., Raijmakers, M. E. J., & Crone, E. A. (2008). Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback-based learning across development. Journal of Neuroscience, 28(38), 9495–9503.
van Leijenhorst, L., Crone, E., & Bunge, S. (2006). Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia, 44(11), 2158–2170. doi:10.1016/j.neuropsychologia.2006.02.002
Velhagen, K., & Broschmann, D. (2005). Tafeln zur Prüfung des Farbensinns [Table of tests for color blindness]. Stuttgart: Thieme.
Waszak, F., Li, S., & Hommel, B. (2010). The Development of Attentional Networks: Cross-Sectional Findings From a Life Span Sample. Developmental Psychology, 46(2), 337–349. doi:10.1037/a0018541
Wechsler, D. (1958). The measurement and appraisal of adult intelligence (4th ed.). Baltimore: Williams & Wilkins.
Weil, R. S., Furl, N., Ruff, C. C., Symmonds, M., Flandin, G., Dolan, R. J., Driver, J., & Rees, G. (2010). Rewarding feedback after correct visual discriminations has both general and specific influences on visual cortex. Journal of Neurophysiology, 104, 1746.
Wolfe, J. M. (1994). Guided search 2.0 A revised model of visual search. Psychonomic Bulletin & Review, 1(2), 202–238.
Wolfe, J. (2013). When is it time to move to the next raspberry bush? Foraging rules in human visual search. Journal of Vision, 13, 1–17.
Yantis, S., Anderson, B.A., Wampler, E.K., & Laurent, P.A. (in press). Reward and attentional control in visual search. In M. Todd & J. Flowers (Eds.) Nebraska Symposium on Motivation (Vol. 59). The Influence of Attention, Learning, and Motivation on Visual Search. Lincoln, NE: University of Nebraska Press
Acknowledgments
We thank the student assistants, as well as Louisa D'Errico and Christoph Kaiser, for help with data collection. This project was conducted in the Neuromodulation of Lifespan Cognition Project while the authors worked at the Max Planck Institute for Human Development. In part, the project was also funded by a grant from the German Ministry of Education and Research to S.-C. Li (BMB/FKZ: 01GQ0913). Viola S. Störmer was supported by the International Max Planck Research School: The Life Course: Evolutionary and Ontogenetic Dynamics and by a Marie Curie fellowship (EU Grant PIOF-GA-2012-329920).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Störmer, V., Eppinger, B. & Li, SC. Reward speeds up and increases consistency of visual selective attention: a lifespan comparison. Cogn Affect Behav Neurosci 14, 659–671 (2014). https://doi.org/10.3758/s13415-014-0273-z
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-014-0273-z