Abstract
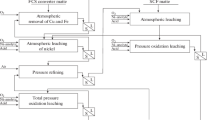
Spent acid is produced in ever-larger quantities in the sulfuric-acid washing of naphthalene. Since 2005, its production has increased by 15%, to more than 90 m3 per month. In addition, its content of organic impurities has doubled, on account of deterioration in the naphthalene fraction and the discontinuation of pyridine removal. The naphthalene content in the organic impurities has fallen by a factor of 1.5, with increase in the content of heavy pyridine bases. The removal of pyridine in polymerizational washing increased the consumption of sulfuric acid by 0.015 t/t of naphthalene. The spent acid is collected in a tank and sent for processing in the sulfate department of byproduct-capture shop 2. Analysis of the distribution of organic impurities along the supply and processing chain shows that, in comparison with 2005, the residue in the tanks after acid discharge is reduced by a factor of 1.3; the content of organic impurities remaining in the tank is reduced by 2.5. The effectiveness of partial regeneration of the spent acid in the tank is a third as much as in 2005. In all, 1.2% of the naphthalene is lost with the acid. More than 61% of the naphthalene settles in the tanks; ~9% is in the store within byproduct-capture shop 2; 28% is in the acidic tar; and 0.6% is in ammonium sulfate. Each month, >10 t of heavy pyridine bases are discharged with the spent acid: 16% remains in the tanks; >65% is in the ammonium sulfate. Since 2005, the content of pyridine bases in the ammonium sulfate has increased sevenfold. This may be attributed to increased supply of heavy pyridine bases with the spent acid and to the discontinuation of the separation of light pyridine bases from the mother liquor in the byproduct-capture shop. To reduce the output of spent acid, hydrogen peroxide is added to the concentrated sulfuric acid. The peroxymonosulfuric acid formed improves the polymerizational washing, with decrease in sulfuric-acid consumption by 15% and increase in the removal of indole from naphthalene by 18%. The consumption of hydrogen peroxide is 0.02 kg/t of naphthalene. With increase in density of the tar in the naphthalene fraction, the content of the absorbing fraction increases. Accordingly, the quality of the naphthalene fraction may be boosted by reducing the phenol content in the phenol fraction.
Similar content being viewed by others
References
Yakusheva, E.A., Bychkova, N.G., and Zotov, A.S., Reducing the content of organic impurities in the spent acid after the polymerization of naphthalene, Koks Khim., 2005, no. 7, pp. 22–25.
Zykov, D.D. and Pats, B.M., Naftalin kok- sokhimicheskii (Naphthalene as a Coke Byproduct), Moscow Metallurgiya, 1981.
Nekrasov, B.V., Kurs obshchei khimii (Course in Gen- eral Chemistry), Moscow GONTI, 1954.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Yakusheva, V.V. Zvonarev, I.V. Zverev, 2015, published in Koks i Khimiya, 2015, No. 6, pp. 36–39.
About this article
Cite this article
Yakusheva, E.A., Zvonarev, V.V. & Zverev, I.V. Processing the spent acid from the sulfuric-acid washing of naphthalene in coke production at OAO EVRAZ NTMK. Coke Chem. 58, 220–223 (2015). https://doi.org/10.3103/S1068364X15060113
Received:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068364X15060113