Abstract
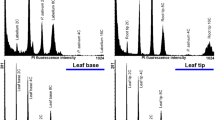
Endopolyploidy is typical and wide-spread in the Orchidaceae. In this study, an attempt was made to quantify the ploidy of mature callus and mature protocorm-like bodies (PLBs) of nine hybrid Cymbidium cultivars cultured in vitro, as well as different flower parts (dorsal sepal, petal, lateral sepal, labellum, pedicel, column, anther cap, stigmatic surface) and youngest leaf of Phalaenopsis and Paphiopedillum and in leaf tissues of in vitro Spathiphyllum and Syngonium plants. Polyploidy was detected in in vitro cultures, primarily in PLBs, ant to a limited extent in floral tissue: 8C was detected in the pedicel and column, but never exceeding 3%. This study expands on the number and breadth of cases of endopolyploidy in orchids, with novel information about Paphiopedillum. These findings may be useful for better understanding developmental processes in vitro or in planta, and may be the first step to revealing evolutionary or adaptive mechanisms of survival of orchids in response to environmental or abiotic stresses.
Similar content being viewed by others
Abbreviations
- PGR:
-
plant growth regulator
- PLB:
-
protocorm-like body
- TC medium:
-
Teixeira Cymbidium medium
- TCL:
-
thin cell layer
References
Adams K.L. & Wendel J.F. 2005. Polyploidy and genome evolution in plants. Curr. Opinion Plant Biol. 8: 135–141.
Alvarez M.R. 1968. Quantitative changes in nuclear DNA accompanying postgermination embryonic development in Vanda (Orchidaceae). Amer. J. Bot. 55: 1036–1041.
Arrigo N. & Barker M.S. 2012. Rarely successful polyploids and their legacy in plant genomes. Curr. Opinion Plant Biol. 15: 140–146.
Barow M., Meister A. 2003. Endopolyploidy in seed plants is differently correlated to systematics, organ, life strategy and genome size. Plant Cell Env. 26: 571–584.
Barow M. 2006. Endopolyploidy in seed plants. BioEssay 28: 271–281.
Bryant J. & Francis D. 2001. Plant growth regulators and the control of S-phase. In: Francis D. (ed.), The Plant Cell Cycle and its Interfaces. Sheffield Academic Press, Sheffield.
Chen W.H., Tang C.Y. & Kao Y.L. 2009. Ploidy doubling by in vitro culture of excised protocorms or protocorm-like bodies in Phalaenopsis species. Plant Cell Tissue Organ Cult. 98: 229–238.
Chen W.H., Tang C.Y., Lin T.Y., Weng Y.C. & Kao Y.L. 2011. Changes in the endopolyploidy pattern of different tissues in diploid and tetraploid Phalaenopsis aphrodite subsp. formosana (Orchidaceae). Plant Sci. 181: 31–38.
Comai L. 2005. The advantages and disadvantages of being polyploidy. Nature Rev. Genetics 6: 836–846.
De Veylder L., Larkin J.C. & Schnittger A. 2011. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 16: 624–634.
Fujii K., Kawano M. & Kako S. 1999. Effects of benzyladenine and α-naphthalineacetic acid on the formation of protocorm like bodies (PLBs) from explants of outer tissue of Cymbidium PLBs cultured in vitro. J. Jpn. Soc. Hortic. Sci. 68: 35–40.
Fukai S., Hasegawa A. & Goi M. 2002. Polysomaty in Cymbidium. HortScience 37: 1088–1091.
Jackson S. & Chen Z.J. 2010. Genomic and expression plasticity of polyploidy. Curr. Opinion Plant Biol. 13: 153–159.
Jones W.E. & Kuehnle A.R. 1998. Ploidy identification using flow cytometry in tissues of Dendrobium species and cultivars. Lindleyana 13: 11–18.
Kondorosi E., Roudier F. & Gendreau E. 2000. Plant cell-size control: rowing by ploidy? Curr. Opinion Plant Biol. 3: 488–492.
Lee H.C., Chiou D.W., Chen W.H., Markhart A.H., Chen Y.H. & Lin T.Y. 2004. Dynamics of cell growth and endoreduplication during orchid flower development. Plant Sci. 166: 659–667.
Lee H.C., Chen Y.J., Markhart A.H. & Lin T.Y. 2007. Temperature effects on systemic endoreduplication in orchid during floral development. Plant Sci. 172: 588–595.
Lim W.L. & Loh C.S. 2003. Endopolyploidy in Vanda Miss Joaquim (Orchidaceae). New Phytol 159: 279–287.
Lin S., Lee H.C., Chen W.H., Chen C.C., Kao Y.Y., Fu Y.M., Chen Y.H. & Lin T.Y. 2001. Nuclear DNA contents of Phalaenopsis sp. and Doritis pulcherrima. J. Amer. Soc. Hortic. Sci. 126: 195–199.
Mishiba K. & Mii K. 2000. Polysomaty analysis in diploid and tetraploid Portulaca grandiflora. Plant Sci. 156: 213–219.
Nontaswatsri C. & Fukai S. 2005. Regenerative callus of Dianthus ‘Telstar Scarlet’ showing mixoploidy produce diploid plants. Plant Cell Tissue Organ Cult. 83: 351–355.
Park S.Y. & Paek K.Y. 2006. Endoreduplication pattern of somatic embryos and variants occurrence affected by preexisted endoreduplicated cells in Doritaenopsis. Korean J. Plant Biotechnol. 33: 297–302.
Park S.Y., Yeung E.C. & Paek K.Y. 2010. Endoreduplication in Phalaenopsis is affected by light quality from light-emitting diodes during somatic embryogenesis. Plant Biotechnol. Rep. 4: 303–309.
Teixeira da Silva J.A. 2012. New basal media for protocorm-like body and callus induction of hybrid Cymbidium. J. Fruit Ornamental Plant Res. 20: 127–133.
Teixeira da Silva J.A. & Dobránszki J. 2013. How timing of sampling can affect the outcome of the quantitative assessment of plant organogenesis. Sci. Hortic. 159: 59–66.
Teixeira da Silva J.A., Giang D.T.T. & Tanaka M. 2006. Novel photoautotrophic micropropagation of Spathiphyllum. Photosynthetica 44: 53–61.
Teixeira da Silva J.A. & Tanaka M. 2006. Embryogenic callus, PLB and TCL paths to regeneration in hybrid Cymbidium (Orchidaceae). J. Plant Growth Reg. 25: 203–210.
Teixeira da Silva J.A. & Tanaka M. 2009. Culture vessel affects hybrid Cymbidium protocorm-like body and callus formation. Floriculture Ornamental Biotech. 3: 53–55.
Teixeira da Silva J.A., Yam T., Fukai S., Nayak N. & Tanaka M. 2005. Establishment of optimum nutrient media for in vitro propagation of Cymbidium Sw. (Orchidaceae) using protocorm-like body segments. Prop. Ornamental Plants 5: 129–136.
Vacin E. & Went F.W. 1949. Some pH changes in nutrient solutions. Bot. Gaz. 110: 605–613.
Valente P., Tao W. & Verbelen J.P. 1998. Auxins and cytokinins control DNA endoreduplication and deduplication in single cells of tobacco. Plant Sci. 134: 203–215.
Yang M. & Loh C.S. 2004. Systemic endopolyploidy in Spathoglottis plicata (Orchidaceae) development. BMC Cell Biol. 5: 33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Teixeira da Silva, J.A., Giang, D.T.T., Dobránszki, J. et al. Ploidy analysis of Cymbidium, Phalaenopsis, Dendrobium and Paphiopedillum (Orchidaceae), and Spathiphyllum and Syngonium (Araceae). Biologia 69, 750–755 (2014). https://doi.org/10.2478/s11756-014-0370-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-014-0370-z