Abstract
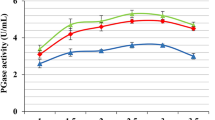
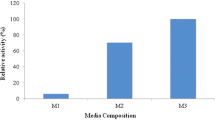
An acidic polygalacturonase (PG) secreted by Rhizopus oryzae MTCC-1987 in submerged fermentation condition has been purified to electrophoretic homogeneity using ammonium sulphate fractionation and anion exchange chromatography on diethylaminoethyl cellulose. The purified enzyme gave a single protein band in sodium dodecyl sulphatepolyacrylamide gel electrophoresis analysis with a molecular mass corresponding to 75.5 kDa. The K m and k cat values of the PG were 2.7 mg/mL and 2.23 × 103 s−1, respectively, using citrus polygalacturonic acid as the substrate. The optimum pH of the purified PG was 5.0 and it does not loose activity appreciably if left for 24 hours in the pH range from 5.0 to 12.0. The optimum temperature of purified enzyme was 50°C and the enzyme does not loose activity below 30°C if exposed for two hours. The purified enzyme showed complete inhibition with 1 mM Ag+, Hg2+ and KMnO4, while it was stimulated to some extent by Co2+. The purified PG exhibited retting of Crotalaria juncea fibre in absence of ethylenediaminetetraacetic acid.
Similar content being viewed by others
Abbreviations
- DEAE:
-
diethylaminoethyl
- DNSA:
-
3,5-dinitrosalicylic acid
- EDTA:
-
ethylenediaminetetraacetic acid
- PG:
-
polygalacturonase
- PGA:
-
polygalacturonic acid
- SDS-PAGE:
-
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- TLC:
-
thin layer chromatography
References
Barense R.I., dos S.C. Chellegatti M.A., Fonseca M.J. & Said S. 2001. Partial purification and characterization of exopolygalacturonase II and III of Penicillium frequentans. Braz. J. Microbiol. 32: 327–330.
Devi N.A. & AppuRao A.G. 1996. Fractionation, purification, and preliminary characterization of polygalacturonases produced by Aspergillus carbonarius. Enzyme Microb. Technol. 18: 59–65.
Engel P.C. 1977. Enzyme Kinetics. A Steady State Approach. Chapman and Hall, London.
Foda M.S., Rizk I.R.S., Gibriel A.Y & Basha A.Y. 1984. Biochemical properties of polygalacturonase, produced by Aspergillus aculeatus and Mucor pusillus. Zentralblatt für Mikrobiologie 139: 463–469.
Henriksson G., Akin D.E., Rigsby L.L., Patel N. & Eriksson K.E.L. 1997. Influence of chelating agents and mechanical pretreatment on enzymatic retting of flax. Tex. Res. J. 67: 829–836.
Henriksson G., Akin D.E., Slomczynski D. & Eriksson K.E.L. 1999. Production of highly efficient enzymes for flax retting by Rhizomucor pusillus. J. Biotechnol. 68: 115–123.
Jacob N., Asha Poorna C. & Prema P. 2008. Purification and partial characterization of polygalacturonase from Streptomyces lydicus. Bioresour. Technol. 99: 6697–6701.
Jayani S.R., Shivalika S. & Gupta R. 2005. Microbial pectinolytic enzymes: a review. Process Biochem. 40: 2931–2944.
Kaur G., Kumar S. & Satyanarayana T. 2004. Production, characterization and application of a thermostable polygalacturonase of a thermophilic mould Sporotrichum thermophile Apinis. Bioresour. Technol. 94: 239–243.
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Manjon A., Iborra J.L., Romero C. & Canovas M. 1992. Properties of pectinesterase and endo-d-polygalacturonase coimmobilized in a porous glass support. Appl. Biochem. Biotechnol. 37: 19–31.
Markovic O. & Janecek S. 2001. Pectin degrading glycoside hydrolases of family 28: sequence-structural features, specificities and evolution. Protein Eng. 14: 615–631.
Martin N., De Souza S.R., Da Silva R. & Gomes E. 2004. Pectinase production by fungal strains in solid-state fermentation using agro-industrial bioproduct. Braz. Arch. Biol. Technol. 47: 813–819.
Mertens J.A., Burdick R.C. & Rooney A.P. 2008. Identification, biochemical characterisation and evolution of the Rhizopus oryzae 99–880 polygalacturonase family. Fungal Genet. Biol. 45: 1616–1624.
Mertens J.A & Bowman M.J. 2011. Expression and characterization of fifteen Rhizopus oryzae 99–880 polygalacturonase enzymes in Pichia pastoris. Curr. Microbiol. 62: 1173–1178.
Miller G.L. 1959. Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal. Chem. 31: 426–428.
Molina S.M.G., Pelissari F.A. & Vitorello C.B.M. 2001. Screening and genetic improvement of pectinolytic fungi for degumming of textile fibres. Braz. J. Microbiol. 32: 320–326.
Niture S.K. 2008. Comparative biochemical and structural characterizations of fungal polygalacturonases. Biologia 63: 1–19.
Ortega N., De Diego S., Rodriguez-Nogales J.M., Perez-Mateos M. & Busto M.D. 2004. Kinetic behaviour and thermal inactivation of pectin lyase used in food processing. J. Food Sci. Technol. 39: 631–639.
Parenicova L., Benen J.A., Kester H.C. & Visser J. 1998. pgaE encodes a fourth member of the endopolygalacturonase gene family from Aspergillus niger. Eur. J. Biochem. 251: 72–80.
Parenicova L., Benen J.A., Kester H.C. & Visser J. 2000. pgaA and pgaB encode two constitutively expressed endopolygalacturonases of Aspergillus niger. Biochem. J. 345: 637–644.
Payasi A., Sanwal R. & Sanwal G.G. 2008. Microbial pectate lyases: characterization and enzymological properties. World J. Microbiol. Biotechnol. 25: 1–14.
Pedrolli D.B., Monteiro A.C., Gomes E. & Carmona E.C. 2009. Pectin and pectinases: production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnol. J. 3: 9–18.
Rao M.N., Kembhavi A.A. & Pant A. 1996. Implication of tryptophan and histidine in the active site of endo-polygalacturonase from Aspergillus ustus: elucidation of the reaction mechanism. Biochim. Biophys. Acta. 1296: 167–173.
Rihouey C., Jauneau A., Cabin-Flaman A., Demarty M., Lefevre F. & Morvan C. 1995. Calcium and acidic pectin distribution in flax cell walls: evidence for different kinds of linkages in the cell junction and middle lamella of the cortical parenchyma of flax hypocotyl. Plant Physiol. Biochem. 33: 497–508.
Saito K., Takakuwa N. & Oda Y. 2004. Purification of the extracellular pectinolytic enzyme from the fungus Rhizopus oryzae NBRC 4707. Microbiol. Res. 159: 83–86.
Sakai T., Sakamoto T., Hallaert J. & Vandamme E.J. 1993. Pectin, pectinase and protopectinase: production, properties and applications. Adv. Appl. Microbiol. 39: 213–294.
Sakamoto T., Bonnin E., Quemener B. & Thibault J.F. 2002. Purification and characterisation of two exo-polygalacturonases from Aspergillus niger able to degrade xylogalacturonan and acetylated homogalacturonan. Biochim. Biophys. Acta. 1572: 10–18.
Satyanarayana N.G. & Kumar D.S. 2005. Microbial pectic transeliminases. Biotechnol. Lett. 27: 451–458.
Semenova M.V., Grishutin S.G., Gusakov A.V., Okunev O.N. & Sinitsyn A.P. 2003. Isolation and properties of pectinases from the fungus Aspergillus japonicus. Biochemistry (Moscow) 68: 559–569.
Stratilova E., Markovic O., Skrovimova D., Rexova-Benkova L. & Jornvall H. 1993. Pectinase Aspergillus sp. polygalacturonase: multiplicity, divergence and structural patterns linking fungal bacterial and plant polygalacturonases. J. Protein Chem. 12: 15–22.
Tari C., Dogan N. & Gogus N. 2008. Biochemical and thermal characterization of crude exo-polygalacturonase produced by Aspergillus sojae. Food Chem. 111: 824–829.
Thakur A., Pahwa R., Singh S. & Gupta R. 2010. Production, purification, and characterization of polygalacturonase from Mucor circinelloides ITCC 6025. Enzyme Res. Article ID 170549.
Torres E.F., Sepulveda T.V. & Gonzalez G.V. 2006. Production of hydrolytic depolymerising pectinases. Food Technol. Biotechnol 44: 221–227.
Vazquez C., Patino B. & Martinez M.J. 1993. Purification and characterization of an exopolygalacturonase produced by Fusarium oxysporum f. sp. radicis lycopersici. FEMS Microbiol. Lett. 110:191–196.
Voragen A.G.J., Conen G.J., Verhof R.P. & Schols H.A. 2009. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 20: 263–275.
Yadav S., Yadav P.K., Yadav D. & Yadav K.D.S. 2009. Pectin lyase: a review. Process Biochem. 44: 1–10.
Zhang J., Henriksson H., Szabo I.J., Henriksson G. & Johansson G. 2005. The active component in the flax retting system of the zygomycetes Rhizopus oryzae sb. is a family 28 polygalacturonase. J. Ind. Microbiol. Biotechnol. 32: 431–438.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, S., Anand, G., Dubey, A.K. et al. Purification and characterization of an exo-polygalacturonase secreted by Rhizopus oryzae MTCC 1987 and its role in retting of Crotalaria juncea fibre. Biologia 67, 1069–1074 (2012). https://doi.org/10.2478/s11756-012-0122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-012-0122-x