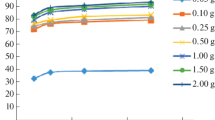
Abstract
A few non-conventional humate sorbents, i.e. iron humate (FeH), aluminium humate (AlH), calcium humate (CaH), magnesium humate (MgH), and zinc humate (ZnH), were prepared from a commercial product Fortehum L/K (Humatex, Bílina, Czech Republic). The metal content in humates was determined by X-ray fluorescence analysis, the organic elements (C, H, N, and S) were analysed by an Elementar Vario III and the functional groups were determined by classical methods using KBr pellets and diffuse reflection infrared spectroscopy (DRIFTS). FeH, AlH, and ZnH were tested as sorbents for the removal of inorganic or organic pollutants (metals, inorganic ions, dyes, and chlorophenols) from waste water. Sorption properties decreased in order: ZnH, AlH, FeH. CaH and MgH are partly soluble and therefore they are not usable as sorbents. However, their ion-exchange abilities for heavy metals are excellent which makes them usable for phytoremediation and bioremediation.
Similar content being viewed by others
References
Ahmad, M., Usman, A. R. A, Lee, S. S., Kim, S. C., Joo, J. H., Yang, J. E., & Ok, Y. S. (2012). Eggshell and coral wastes as low cost sorbents for the removal of Pb2+, Cd2+ and Cu2+ from aqueous solutions. Journal of Industrial and Engineering Chemistry, 18, 198–204. DOI: 10.1016/j.jiec.2011.11.013.
Allouche, F. N., Guibal, E., & Mameri, N. (2014). Preparation of a new chitosan-based material and its application for mercury sorption. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 446, 224–232. DOI: 10.1016/j.colsurfa.2014.01.025.
Andini, S., Cioffi, R., Colangelo, F., Montagnaro, F., & Santoro, L. (2008). Adsorption of chlorophenol, chloroaniline and methylene blue on fuel oil fly ash. Journal of Hazardous Materials, 157, 599–604. DOI: 10.1016/j.jhazmat.2008.01.025.
Ariese, F., Swart, K., Morabito, R., Brunori, C., Balzamo, S., Slobodnik, J., Korenková, E., Janoš, P., Wildnerová, M., Hlavay, J., Polyak, K., Fodor, P., & Muntau, H. (2002). Leaching studies of inorganic and organic compounds from fly ash. International Journal of Environmental Analytical Chemistry, 82, 751–770. DOI: 10.1080/0306731031000078.
Babel, S., & Kurniawan, T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. Journal of Hazardous Materials, 97, 219–243. DOI: 10.1016/s0304-3894(02)00263-7.
Chang Chien, S. W., Chen, C. Y., Chang, J. H., Chen, S. H., Wang, M. C., & Mannepalli, M. R. (2010). Sorption of toluene by humic acids derived from lake sediment and mountain soil at different pH. Journal of Hazardous Materials, 177, 1068–1076. DOI: 10.1016/j.hazmat.2010.01.029.
Chassapis, K., Roulia, M., & Nika, G. (2010). Fe(III)-humate complexes from Megalopolis peaty lignite: A novel ecofriendly fertilizer. Fuel, 89, 1480–1484. DOI: 10.1016/j.fuel.2009.10.005.
Čežíková, L., Novák, J., & Janoš, P. (2001). Humic acids from coals of the North-Bohemian coal field. II. Metal-binding capacity under static conditions. Reactive and Functional Polymers, 47, 111–118. DOI: 10.1016/s1381-5148(00)00078-x.
Dąbrowski, A., Podkościelny, P., Hubicki, Z., & Barczak, M. (2005). Adsorption of phenolic compounds by activated carbon-a critical review. Chemosphere, 58, 1049–1070. DOI: 10.1016/j.chemosphere.2004.09.067.
Dulman, V., & Cucu-Man, S. M. (2009). Sorption of some textile dyes by beech wood sawdust. Journal of Hazardous Materials, 162, 1457–1464. DOI: 10.1016/j.jhazmat.2008.06.046.
European Committee for Standardization (2011). European standard: Soil improvers and growing media. Determination of organic matter content and ash. EN 13039. Brussels, Belgium.
Georgi, A., Trommler, U., Reichl, A., & Kopinke, F. D. (2008). Influence of sorption to dissolved humic substances on transformation reactions of hydrophobic organic compounds in water. Part II: Hydrolysis reactions. Chemosphere, 71, 1452–1460. DOI: 10.1016/j.chemosphere.2007.12.001.
Güngör, E. B. Ö., & Bekbölet, M. (2010). Zinc release by humic and fulvic acid as influenced by pH, complexation and DOC sorption. Geoderma, 159, 131–138, DOI: 10.1016/j.geoderma.2010.07.004.
Hamidpour, M., Kalbasi, M., Afyuni, M., Shariatmadari, H., Holm, P. E., & Hansen, H. C. B. (2010). Sorption hysteresis of Cd(II) and Pb(II) on natural zeolite and bentonite. Journal of Hazardous Materials, 181, 686–691. DOI: 10.1016/j.jhazmat.2010.05.067.
Havelcová, M., Mizera, J., Sykorová, I., & Pekař, M. (2009). Sorption of metal ions on lignite and the derived humic substances. Journal of Hazardous Materials, 161, 559–564. DOI: 10.1016/j.jhazmat.2008.03.136.
Janoš, P., Buchtová, H., & Ryznarová, M. (2003). Sorption of dyes from aqueous solutions onto fly ash. Water Research, 37, 4938–4944. DOI: 10.1016/j.watres.2003.08.011.
Janoš, P., Šedivá, M., & Grötschelová, S. (2005). Sorption of basic and acid dyes from aqueous solutions onto oxihumolite. Chemosphere, 59, 881–886. DOI: 10.1016/j.chemosphere.2004.11.018.
Janoš, P., Fedorovič, J., Staňková, P., Grötschelová, S., Rejnek, J., & Stopka, P. (2006). Iron humate as a low-cost sorbent for metal ions. Environmental Technology, 27, 169–181. DOI: 10.1080/09593332708618627.
Janoš, P., Michálek, P., & Turek, L. (2007a). Sorption of ionic dyes onto untreated low-rank coal — oxihumolite: A kinetic study. Dyes and Pigments, 74, 363–370. DOI: 10.1016/j.dyepig.2006.02.017.
Janoš, P., Sypecká, J., Mlčkovskáň, P., & Pilařov, V. (2007b). Removal of metal ions from aqueous solutions by sorption onto untreated low-rank coal (oxihumolite). Separation and Purification Technology, 53, 322–329. DOI: 10.1016/j.seppur.2006.08.004.
Janoš, P., Křiženeck, S., & Madronová, L. (2008). Acid-base titration curves of solid humic acids. Reactive and Functional Polymers, 68, 242–247. DOI: 10.1016/j.reactfunctpolym.2007.09.005.
Janoš, P., Coskun, S., Pilařov Removal of basic (Methylene Blue) and acid (Egacid Orange) dyes from waters by sorption on chemically treated wood shavings. Bioresource Technology, 100, 1450–1453. DOI: 10.1016/j.biortech.2008.06.069.
Janoš, P., Hůla, V., Bradnová, P., Pilařová, V., Šedlbauer, J. (2009b). Reduction and immobilization of hexavalent chromium with coal- and humate-based sorbents. Chemosphere, 75, 732–738. DOI: 10.1016/j.chemosphere.2009.01.037.
Jha, V. K., Matsuda, M., & Miyake, M. (2008). Sorption properties of the activated carbon-zeolite composite prepared from coal fly ash for Ni2+, Cu2+, Cd2+ and Pb2+. Journal of Hazardous Materials, 160, 148–153. DOI: 10.1016/j.jhazmat.2008.02.107.
Kamble, S. P., Mangrulkar, P. A., Bansiwal, A. K., & Rayalu, S. S. (2008). Adsorption of phenol and o-chlorophenol on surface altered fly ash based molecular sieves. Chemical Engineering Journal, 138, 73–83. DOI: 10.1016/j.cej.2007.05.030.
Kuráň, P., Trögl, J., Nováková, J., Pilařováňová, P., Pavlorková, J., Kozler, J., Novák, F., & Popelka, J. (2014). Biodegradation of spilled diesel fuel in agricultural soil: effect of humates, zeolite, and bioaugmentation. The Scientific World Journal, 2014, 642427. DOI: 10.1155/2014/642427.
Kurková, M., Klika, Z., Kliková, Ch., & Havel, J. (2004). Humic acids from oxidized coals: I. Elemental composition, titration curves, heavy metals in HA samples, nuclear magnetic resonance spectra of HAs and infrared spectroscopy. Chemosphere, 54, 1237–1245. DOI: 10.1016/j.chemosphere.2003.10.020.
Kyziol, J., Twardowska, I., & Schmitt-Kopplin, Ph. (2006). The role of humic substances in chromium sorption onto natural organic matter (peat). Chemosphere, 63, 1974–1982, DOI: 10.1016/j.chemosphere.2005.09.042.
Lipczynska-Kochany, E., & Kochany, J. (2009). Effect of humate on biological treatment of wastewater containing heavy metals. Chemosphere, 77, 279–284. DOI: 10.1016/j.chemosphere.2009.07.036.
Li, B. Z., Sun, K. Q., Guo, Y. B., Tian, J. P., Xue, Y. B., & Sun, D. K. (2013). Adsorption kinetics of phenol from water on Fe/AC. Fuel, 110, 99–106. DOI: 10.1016/j.fuel.2012.10.043.
Liu, X., & Pinto, N. G. (1997). Ideal adsorbed phase model for adsorption of phenolic compounds on activated carbon. Carbon, 35, 1387–1397. DOI: 10.1016/s0008-6223(97)00092-4.
Lyngsie, G., Borggaard, O. K., & Hansen, H. C. B. (2014). A three-step test of phosphate sorption efficiency of potential agricultural drainage filter materials. Water Research, 51, 256–265. DOI: 10.1016/j.watres.2013.10.061.
Madronová, L. (Ed.) (2011). Humic acids from raw materials of the Czech Republic. New York, NY, USA: Nova Science Publishers.
Neves Fernandes, A., Policiano Almeida, C. A., Debacher, N. A., & de Souza Sierra, M. M. (2010). Isotherm and thermodynamic data of adsorption of methylene blue from aqueous solution onto peat. Journal of Molecular Structure, 982, 62–65. DOI: 10.1016/j.molstruc.2010.08.006.
Nurchi, V. M., & Villaescusa, I. (2012). Sorption of toxic metal ions by solid sorbents: A predictive speciation approach based on complex formation constants in aqueous solution. Coordination Chemistry Reviews, 256, 212–221. DOI: 10.1016/j.ccr.2011.09.002.
Lubal, P., Širok and complexation properties of humic acids: Study of complexation of Czech humic acids with metal ions. Talanta, 47, 401–412. DOI: 10.1016/s0039-9140(98)00143-x. x
Rodríguez, A., García, J., Ovejero, G., & Mestanza, M. (2009). Adsorption of anionic and cationic dyes on activated carbon from aqueous solutions: Equilibrium and kinetics. Journal of Hazardous Materials, 172, 1311–1320. DOI: 10.1016/j.jhazmat.2009.07.138.
Salleh, M. A. M., Mahmoud, D. K., Karim, W. A. W. A., & Idris, A. (2011). Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination, 280, 1–13. DOI: 10.1016/j.desal.2011.07.019.
Turgay, O. C., Erdogan, E. E., & Karaca, A. (2010). Effect of humic deposit (leonardite) on degradation of semivolatile and heavy hydrocarbons and soil quality in crude-oilcontaminated soil. Environmental Monitoring and Assessment, 170, 45–58. DOI: 10.1007/s10661-009-1213-1.
Yi, J. Z., & Zhang, L. M. (2008). Removal of methylene blue dye from aqueous solution by adsorption onto sodium humate/polyacrylamide/clay hybrid hydrogels. Bioresource Technology, 99, 2182–2186. DOI: 10.1016/j.biortech.2007.05.028.
Yousef, R. I., & El-Eswed, B. (2009). The effect of pH on the adsorption of phenol and chlorophenols onto natural zeolite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 334, 92–99. DOI: 10.1016/j.colsurfa.2008.10.004.
Zeledón-Toruño, Z., Lao-Luque, C., & Solé-Sardans, M. (2005). Nickel and copper removal from aqueous solution by an immature coal (leonardite): effect of pH, contact tome and water hardness. Journal of Chemical Technology and Biotechnology, 80, 649–656. DOI: 10.1002/jctb.1243.
Zhang, Q., Zhao, L., Dong, Y. H., & Huang, G. Y. (2012). Sorption of norfloxacin onto humic acid extracted from weathered coal. Journal of Environmental Management, 102, 165–172. DOI: 10.1016/j.jenvman.2011.12.036.
Zhuo, L., Li, H., Cheng, F. Q., Shi, Y. L., Zhang, Q. H., & Shi, W. Y. (2012). Co-remediation of cadmium-polluted soil using stainless slag and ammonium humate. Environmental Science and Pollution Research, 19, 2842–2848. DOI: 10.1007/s11356-012-0790-7.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kříženecká, S., Hejda, S., Machovič, V. et al. Preparation of iron, aluminium, calcium, magnesium, and zinc humates for environmental applications. Chem. Pap. 68, 1443–1451 (2014). https://doi.org/10.2478/s11696-014-0586-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0586-y