Abstract
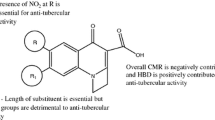
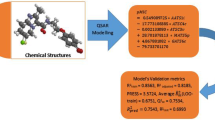
Correlation analysis and, in particular, artificial neural networks (ANN) were used to predict the anti-mycobacterial activity of substituted 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-diones (PBODs) by quantitative structure — activity relationship (QSAR) calculations. Initially, sixty-four derivatives were synthesised and biologically tested; ten further derivatives were proposed for future synthesis on the basis of the prediction results. The biological activity was originally expressed by minimum inhibitory concentration (MIC) against Mycobacterium tuberculosis; however, its transformed pMIC form was found to be more informative. Theoretical molecular descriptors of several types were selected to establish a primary drug model of the species which was expected to exhibit a substantial anti-mycobacterial effect. Lipophilicity and solubility indices, several basic molecular properties, quantum chemistry quantities as well as 1H and 13C NMR chemical shifts, were employed as the descriptors, enabling a very successful prediction of the pMIC values. The utilisation of in silico variables and simulated NMR data is highly advantageous in the first phase of the drug design, as they permit prediction of the compounds with a high expected activity, minimising the risk of synthesising less active species. The MIC values predicted at less than 4 μmol L−1 for six of the ten compounds suggested for further synthesis are better than the best value for the original set of compounds.
Similar content being viewed by others
References
ACD/Labs (2003). ACD/PhysChem, Version 7.0 [computer software]. Toronto, Canada: Advanced Chemistry Development.
Bishop, C. M. (1995). Neural networks for pattern recognition. Oxford, UK: Oxford University Press.
Corbett, E. L., Churchyard, G. J., Hay, M., Herselman, P., Clayton, T., Williams, B., Hayes, R., Mulder, D.,& De Cock, K. M. (1999). The impact of HIV infection on Mycobacterium kansasii disease in South African gold miners. American Journal of Respiratory and Critical Care Medicine, 160, 10–14.
Devillers, J. (Ed.) (1996). Neural networks in QSAR and drug design (Series: Principles of QSAR and drug design). London, UK: Academic Press.
Dewar, M. J. S., Zoebisch, E.G., Healy, E. F.,& Stewart, J. J. P. (1985). Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. Journal of the American Chemical Society, 107, 3902–3909. DOI: 10.1021/ja00299a024.
Ďurčeková, J., Mocák, J., Lehotay, J., Čižmárik, J., & Boronová, K. (2010). Chemometrical study of the anaesthetical activity of alkoxyphenylcarbamic acid esters. Die Pharmazie — An International Journal of Pharmaceutical Sciences, 65, 169–174. DOI: 10.1691/ph.2010.9691.
Ďurčeková, J., Mocák, J., Boronová, K.,& Balla, J. (2011). Effect of the statin therapy on biochemical laboratory tests—A chemometrics study. Journal of Pharmaceutical and Biomedical Analysis, 54, 141–147. DOI: 10.1016/j.jpba.2010. 07.047.
Ďurčeková, T., Boronová, K., Mocák, J., Lehotay, J.,& Čižmárik, J. (2012). QQSRR models for potential local anaesthetic drugs using high performance liquid chromatography. Journal of Pharmaceutical and Biomedical Analysis, 59, 209–216. DOI: 10.1016/j.jpba.2011.09.035.
El-Sadr, W. M., Burman, W. J., Grant, L. B., Matts, J. P., Hafner, R., Crane, L., Zeh, D., Gallagher, B., Mannheimer, S. B., Martinez, A.,& Gordin, F. (2000). Discontinuation of prophylaxis against Mycobacterium avium complex disease in HIV-infected patients who have a response to antiretroviral therapy. The New England Journal of Medicine, 342, 1085–1092. DOI: 10.1056/nejm200004133421503.
Frieden, T. R., Sterling, T. R., Munsiff, S. S., Watt, C. J.,& Dye, C. (2003). Tuberculosis. Lancet, 362, 887–899. DOI: 10.1016/s0140-6736(03)14333-4.
Lin, L. (1989). A concordance correlation coefficient to evaluate reproducibility. Biometrics, 45, 255–268. DOI: 10.2307/2532051.
Marras, T. K., Morris, A., Gonzalez, L. C.,& Daley, C. L. (2004). Mortality prediction in pulmonary Mycobacterium kansasii infection and human immunodeficiency virus. American Journal of Respiratory and Critical Care Medicine, 170, 793–798.
Mitha, M., Naicker, P.,& Taljaard, J. (2011). Cutaneous Mycobacterium kansasii infection in a patient with AIDS post initiation of antiretroviral therapy. The Journal of Infection in Developing Countries, 5, 553–555.
Mohan Raju, M., Srivastava, R. K., Bisht, D. C. S., Sharma, H. C., & Kumar, A. (2011). Development of artificial neuralnetwork-based models for the simulation of spring discharge. Advances in Artificial Intelligence, 2011, 686258. DOI: 10.1155/2011/686258.
Myers, J. L., & Well, A. D. (2003). Research design and statistical analysis (2nd ed.). Mahwah, NJ, USA: Lawrence Erlbaum Associates.
Nemeček, P., Ďurčekovák, J., Waisser, K., & Lehotay, J. (2008). Utilization of artificial neural networks for prediction of biological activity in novel QSAR approach. In P. Glavič, & D. Brodnjak-Vončina (Eds.), Proceedings of the Slovenian Chemical Days, September 25–26, 2008. Maribor, Slovenia: Slovensko Kemijsko Družstvo.
Nemeček, P., Ďurčeková, T., Mocák, J.,& Waisser, K. (2009). Chemometrical analysis of computed QSAR parameters and their use in biological activity prediction. Chemical Papers, 63, 84–91. DOI: 10.2478/s11696-008-0089-9.
Niculescu, S. P. (2003). Artificial neural networks and genetic algorithms in QSAR. Journal of Molecular Structure (THEOCHEM), 622, 71–83. DOI: 10.1016/s0166-1280(02) 00619-x.
Patterson, D.W. (1996). Artificial neural networks: Theory and applications (Prentice Hall series in advanced communications). Singapore: Prentice Hall.
Petrlíková, E., Waisser, K., DoleŽal, R., Holy, P., Gregor, J., Kuneš, J.,& Kaustová, J. (2011). Antimycobacterial 3-phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-ones and 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dithiones substituted on phenyl and benzoxazine moiety in position 6. Chemical Papers, 65, 352–366. DOI: 10.2478/s11696-011-0020-7.
Shepherd, A. J. (1997). Second-order methods for neural networks: Fast and reliable training methods for multi-layer perceptrons (Series: Perspectives in neural computing). New York, NY, USA: Springer.
StatSoft (1984). STATISTICA, Version 7.0 [computer software]. Tulsa, OK, USA: StatSoft.
Tetko, I. V., Gasteiger, J., Todeschini, R., Mauri, A., Livingstone, D., Ertl, P., Palyulin, V. A., Radchenko, E.V., Zefirov, N. S., Makarenko, A. S., Tanchuk, V. Y., & Prokopenko, V. V. (2005). Virtual computational chemistry laboratory — design and description. Journal of Computer-Aided Molecular Design, 19, 453–463. DOI: 10.1007/s10822-005-8694-y.
Tomioka, H.,& Namba, K. (2006). Development of antituberculous drugs: Current status and future prospects. Kekkaku, 81, 753–774.
VCCLAB (2005). Virtual computational chemistry laboratory. Retrieved from http://www.vcclab.org
Waisser, K., Kubicová, L., Klimešová, Z. (1993). New groups of potential antituberculotics: 3-aryl-2H,4H-benz[e][1,3]oxazine-2,4-diones. Comparison of the Topliss approach with regression analysis. Collection of Czechoslovak Chemical Communications, 58, 2977–2982. DOI: 10.1135/cccc19932977.
Waisser, K., Hladuvková, J., Gregor, J., Rada, T., Kubicová, L., Klimešová, V., & Kaustová, J. (1998). Relationships between the chemical structure of antimycobacterial substances and their activity against atypical strains. Part 14: 3-aryl-6,8-dihalogeno-2H-1,3-benzoxazine-2,4(3H)-diones. Archiv der Pharmazie, 331, 3–6. DOI: 10.1002/(SICI)1521-4184(199801) 331:1〈3::AID-ARDP3〉3.0.CO;2-2.
Waisser, K., Macháček, M., Dostál, H., Gregor, J., Kubicová, L., Klimešovš, J., Palát, K., Jr., Hladůvkov, J., Kaustová, J.,& Möllmann, U. (1999). Relationships between the chemical structure of substances and their antimycobacterial activity against atypical strains. Part 18. 3-Phenyl-2H-1,3-benzoxazine-2,4(3H)-diones and isosteric 3-phenylquinazoline-2,4(lH,3H)-diones. Collection of Czechoslovak Chemical Communications, 64, 1902–1924. DOI: 10.1135/cccc19991902.
Waisser, K., Hladůvková, J., Holy, P., Macháčmk, M., Karajannis, P., Kubicová, L., Klimešová, V., Kuneš, J., & Kaustová, J. (2001). 2H-1,3-Benzoxazine-2,4(3H)-diones substituted in position 6 as antimycobacterial agents. Chemical Papers, 55, 323–334.
Waisser, K., Bureš, O., Holy, P., Kuneš, J., Oswald, R., Jirásková, L., Pour, M., Klimešovát, K., Jr., Kaustová, J., Dahse, H. M.,& Möllmann, U. (2003). Antimycobacterial 3-aryl-2H-1,3-benzoxazine-2,4(3H)-diones. Pharmazie, 58, 83–94. DOI: 10.1002/chin.200324133.
Waisser, K., Matyk, J., Divišováková, P., Kuneš, J., Klimešová, J., Möllmann, U., Dahse, H. M.,& Miko, M. (2006). The oriented development of antituberculotics: Salicylanilides. Archiv der Pharmazie, 339, 616–620. DOI: 10.1002/ardp.200600093.
Waisser, K., Matyk, J., Kuneš, J., Doležal, R., Kaustová, J.,& Dahse, H. M. (2008). Highly active potential antituberculotics: 3-(4-Alkylphenyl)-4-thioxo-2H-1,3-benzoxazine-2(3H)-ones and 3-(4-alkylphenyl)-2H-1,3-benzoxazine-2,4(3H)-dihiones substituted in ring-B by halogen. Archiv der Pharmazie, 341, 800–803. DOI: 10.1002/ardp.200800004.
Zupan, J., & Gasteiger, J. (1999). Neural networks in chemistry and drug design (2nd ed.). Weinheim, Germany: Wiley.
Author information
Authors and Affiliations
Corresponding author
Additional information
Professor Mocák passed away on March 1, 2012.
Rights and permissions
About this article
Cite this article
Nemeček, P., Mocák, J., Lehotay, J. et al. Prediction of anti-tuberculosis activity of 3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dione derivatives. Chem. Pap. 67, 305–312 (2013). https://doi.org/10.2478/s11696-012-0278-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-012-0278-4