Abstract
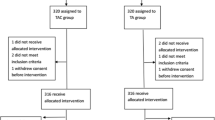
Adjuvant chemotherapy decreases the risk of breast cancer recurrence in patients with breast cancer. In addition, it increases the rate of survival. Therefore, various chemotherapy regimens are administered in the treatment of breast cancer. The efficacy of taxane-based adjuvant chemotherapies has been demonstrated in various trials. This trial was designed to retrospectively evaluate the efficacy of taxane-based chemotherapies in lymph node-positive, early-stage Turkish breast cancer patients. 29 patients receiving TAC regimen and 29 patients receiving AC+P regimen were evaluated. 6 courses of TAC regimen were administered every 3 weeks (docetaxel 75 mg/m2, doxorubicine 50 mg/m2, cyclophosphamide 500 mg/m2). The other patient group was administered AC+P regimen (4 courses of doxorubicin 60mg/m2, cyclophosphamide 600 mg/m2 combination every 2 weeks, followed by paclitaxel 175 mg/m2 for 4 courses every 2 weeks). The 1-year, 2-year and 3-year disease-free survival (DFS) rates were 96.3%, 81.1% and 72.8% respectively. No significant difference was detected in DFS between premenopausal and postmenopausal patients on the taxane regimen (p=0.82). There was no significant difference in DFS between estrogen or progesterone receptor positive and negative patients (p=0.46). Disease-free survival of patients receiving TAC and AC+P adjuvant chemotherapy regimen was compared. The follow-up period of patients on AC+P chemotherapy was longer than those receiving TAC (AC+P mean 38.6±12.8 months, TAC mean 17.1±5.4 months). No significant difference was observed upon evaluation of both treatment arms with respect to DFS (p=0.92). In conclusion, this trial once more demonstrated that taxane-based adjuvant chemotherapy was effective and safe in lymph node-positive, early-stage Turkish breast cancer patients.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687–1717
Hudis C. The best use of adjuvant chemotherapy: New drugs and new use of old drugs. The Breast 2005;14: 570–575
Ring AE, Ellis A. Taxanes in the treatment of early breast cancer. Cancer Treat Rev 2005;31: 618–627
Bria E, Nistico C, Cuppone F, Carlini P, Ciccarese M, Milella M, Natoli G, Terzoli E, Cognetti F, Giannarelli D. Benefit of taxanes as adjuvant chemotherapy for early breast cancer. Cancer 2006;106:2337–2344
Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 2003;21:976–983
Mamounas EP, Bryant J, Lembersky B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node positive breast cancer: results from NSABP B-28. J Clin Oncol 2005,23: 3686–3696
Martin M, Pienkowski T, Mackey J et al. Adjuvant docetaxel for node positive breast cancer. N Engl J Med 2005;352:2302–2313
Crown JP, Francis P, Di Leo A, et al. Docetaxel (T) given concurrently with or sequentially to anthracycline-based (A) adjuvant therapy (adjRx) for patients (pts) with node-positive (N+) breast cancer (BrCa), in comparison with non-T adjRx: First results of the BIF-2-98 Trial at 5-years median follow-up (MFU). Proc Am Soc Clin Oncol 2006; 24: Abstract 519
Sparano JA, Wang M, Martino S, et al. Phase III study of doxorubicin-cyclophosphamide followed by paclitaxel or docetaxel given every 3 weeks or weekly in patients with axillary node positive of high-risk node-negative breast cancer: results of North American Breast Cancer Intergroup Trial E1199. Breast Cancer Res Treat. 2005;94(suppl 1). Abstract 48
Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, Teissier E, Berdah JF, Fabbro M, Valenza B, Herait P, Jehl V, Buyse M. Dose-dense adjuvant chemotherapy in node positive breast cancer: followed by epirubicine/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Annals of Oncology 2007;18:52–57
Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 2006;12:1658–1667
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Alacacioglu, A., Zengel, B. & Denecli, A.G. Taxane-based adjuvant chemotherapy in node positive, early stage Turkish breast cancer patients. cent.eur.j.med 4, 454–458 (2009). https://doi.org/10.2478/s11536-009-0075-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11536-009-0075-9