Abstract
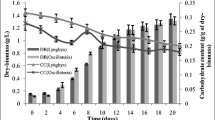
The microalga Porphyridium cruentum (Rhodophyta) has several industrial and pharmaceutical uses, especially for its polysaccharide production. This study aimed to investigate the influence of nitrogen levels as reflected by altered N:P ratios on the production and content of biomass and carbohydrate. N:P molar ratios were altered in batch cultures to range from 1.6 to 50 using the Redfield ratio of 1:16 as reference. Algal growth (estimated as final cell number, biomass concentration and maximum specific growth rate) was negatively affected at low N:P ratios. The optimal N:P ratio for growth was identified at 35–50, with specific growth rates of 0.19 day−1 and maximum cell concentrations of 59·108 cells L−1 and 1.2 g dry weight of biomass L−1. In addition, variation in cell size was seen. Cells with larger diameters were at higher N:P ratios and smaller cells at lower ratios. The cellular carbohydrate content increased under reduced nitrogen availability. However, because accumulation was moderate at the lowest N:P ratio, 0.4 g per g dry weight biomass compared to 0.24 at the Redfield ratio of 16:1, conditions for increased total carbohydrate formation were identified at the N:P ratios optimal for growth. Additionally, carbohydrates were largely accumulated in late exponential to stationary phase.
Similar content being viewed by others
References
Ahern T.J., Katoh S., Sada E., Arachidonic acid production by the red alga Porphyridium cruentum, Biotechnol. Bioeng., 1983, 25, 1057–1070
Oh S.H., Han J.G., Kim Y., Ha J.H., Kim S.S., Jeong M.H., et al., Lipid production in Porphyridium cruentum grown under different culture conditions, J. Biosci. Bioeng., 2009, 108, 429–434
Kathiresan S., Sarada R., Bhattacharya S., Ravishankar G.A., Culture media optimization for growth and phycoerythrin production from Porphyridium purpureum, Biotechnol. Bioeng., 2007, 96, 456–463
Arad S.M., Levy-Ontman O., Red microalgal cellwall polysaccharides: biotechnological aspects, Curr. Opin. Biotechnol., 2010, 21, 358–364
Patel A.K., Laroche C., Marcati A., Ursu A.V., Jubeau S., Marchal L., et al., Separation and fractionation of exopolysaccharides from Porphyridium cruentum, Bioresource Technol, 2012, In Press, idoi: 10.1016/j.biortech.2012.1012.1038
Heaney-Kieras J., Chapman D.J., Structural studies on the extracellular polysaccharide of the red alga, Porhyridium cruentum, Carbohyd. Res., 1976, 52, 169–177
Arad S., Adda M., Cohen E., The potential production of sulfated polysaccharides from Porphyridium, Plant Soil, 1985, 89, 117–127
Becker E.W., Microalgae: biotechnology and microbiology, Cambridge University Press, Cambridge, 1994
John R.P., Anisha G.S., Nampoothiri K.M., Pandey A., Micro and macroalgal biomass: a renewable source for bioethanol, Bioresour. Technol., 2011, 102, 186–193
Kroen W.K., Raynburn W.R., Influence of growth status and nutrients on extracellular polysaccharide synthesis by the soil agla Chlamydomonas mexicana (Chlorophyceae), J. Phycol., 1984, 20, 253–257
Brányiková I., Marsalková B., Doucha J., Brányik T., Bisová K., Zachleder V., et al., Microalgaenovel highly efficient starch producers, Biotechnol. Bioeng., 2011, 108, 766–776
Yao C., Ai J., Cao X., Xue S., Zhang W., Enhancing starch production of a marine green microalga Tetraselmis subcordiformis through nutrient limitation, Bioresour. Technol., 2012, 118, 438–444
Kilham S.S., Kreeger D.A., Goulden C.E., Lynn S.G., Effect of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus, Freshwater. Biol., 1997, 38, 591–596
Lourenco S.O., Lanfer Marquez U.M., Mancini-Filho J., Barbarino E., Aidar E., Changes in biochemical profile of Tetraselmis gracilis I. Comparison of two culture media, Aquaculture, 1997, 148, 153–168
Ramus J., The production of extracellular polysaccharide by unicellular red alga Porphyridium aerugineum, J. Phycol., 1972, 8, 97–111
Arad S.M., Friedman O.D., Rotem A., Effect of nitrogen on polysaccharide production in a Porphyridium sp., Appl. Environ. Microbiol., 1988, 54, 2411–2414
Carstensen J., Henriksen P., Heiskanen A.S., Summer algal blooms in shallow estuaries: Definition, mechanisms, and link to eutrophication, Limnol. Oceanogr., 2007, 52, 370–384
Adda M., Merchuk J.C., Arad S., Effect of nitrate on growth and production of cell-wall polysaccharide by the unicellular red alga Porphyridium, Biomass, 1986, 10, 131–140
Levy I., Gantt E., Development of photosynthetic activity in Porphyridium purpureum (Rhodophyta) following nitrogen starvation, J. Phycol., 1990, 26, 62–68
Redfield A.C., The biological control of chemical factors in the environment, Am. Sci., 1958, 46, 205–221
Klausmeier C.A., Litchman E., Daufresne T., Levin S.A., Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton, Nature, 2004, 429, 171–174
MacIntyre H.L., Cullen J.J., Using cultures to investigate the physiological ecology of microalgae, In: Andersen R.A., Ed., Algal culturing techniques. Elsevier Academic Press, London, UK, 2005, 287–326
Thepenier C., Gudin C., Studies on optimal conditions for polysaccharide production by Porphyridium cruentum, World J. Microbiol. Biotechnol., 1985, 1, 257–268
Vonshak A., Cohen Z., Richmond A., The feasibility of mass cultivation of Porphyridium, Biomass, 1985, 8, 13–25
Andersen R.A., Ed. Algal culturing techniques. Elsevier Academic Press, London, UK, 2005
Tunzi M.G., Chu M.Y., Bain R.C., In vivo fluorescence, extracted fluorescence, and chlorophyll concentrations in algal mass measurements, Water Res., 1974, 8, 623–635
Lavens P., Sorgeloos P., Manual on the production and use of life food for aquaculture, FAO Fisheries Technical Papers T361, FAO, Rome, 1996, ftp://ftp.fao.org/docrep/fao/003/w3732e/w3732e00.pdf
Herbert D., Phipps P.J., Strange R.E., Chemical analysis of microbial cells, In: Norris J.R., Ribons D.W., Eds., Methods in microbiology. Academic Press, London, 1971, 209–344
Lien T., Knutsen G., Phosphate as a control factor in cell division of Chlamydomonas reinhardti, studied in synchronous culture, Exp. Cell. Res., 1973, 78, 79–88
Roessler P.G., Environmental control of glycerolipid metabolism in microalgae: Commercial implications and future research directions, J. Phycol., 1990, 26, 393–399
Young E.B., Beardall J., Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle, J. Phycol., 2003, 39, 897–905
Percival E., Foyle R.A.J., Extracellular polysaccharides of Porphyridium cruentum and Porphyridium aerugineum, Carbohyd. Res., 1979, 72, 165–176
Lien T., Knutsen G., Synchronous cultures of Chlamydomonas reinhardti. Synthesis of repressed and derepressed phosphatase during the life cycle, Biochim. Biophys. Acta., 1972, 287, 154–163
Arrigo K.R., Marine microorganisms and global nutrient cycles, Nature, 2005, 437, 349–355
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Razaghi, A., Godhe, A. & Albers, E. Effects of nitrogen on growth and carbohydrate formation in Porphyridium cruentum . cent.eur.j.biol. 9, 156–162 (2014). https://doi.org/10.2478/s11535-013-0248-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-013-0248-z