Abstract
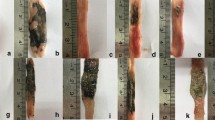
Inflammatory bowel disease (IBD) is a chronic inflammatory condition with an unknown etiology. Nicorandil, a potassium channel opener, has been used for many years for the treatment of angina. Recently, it has been shown that nicorandil possesses some novel traits such as anti-apoptotic, gastroprotective, free radical scavenging, and anti-inflammatory properties. Therefore, we set out to examine the possible beneficial effect of nicorandil in a rat model of IBD. Colitis was induced by rectal administration of 2,4,6-trintrobenzene sulphonic acid (TNBS) into rats. Groups of animals used in this study were sham, control, and exposure to dexamethasone, nicorandil, glibenclamid (a pure adenosine triphosphate sensitive potassium channel (KATP) blocker), or nicorandil plus glibenclamid. Drugs were administered by gavage and animals were sacrificed after 7 days. Biochemical markers, including TNF-α and IL-1β, ferric reducing/antioxidant power (FRAP), myeloperoxidase (MPO) activity and thiobarbitoric acid-reactive substance (TBARS), were measured in the homogenate of colonic tissue. Results indicate that nicorandil significantly reduces macroscopic and histological damage induced by TNBS. Nicorandil diminishes MPO activity and levels of TBARS, TNF-∢, and IL-1β in damaged colonic tissue with a concomitant increase in FRAP value (P<0.01). These effects were not reversed by coadministration of glibenclamide. In conclusion, nicorandil is able to ameliorate experimental IBD with a dose in which it does not show any anti-hypertensive effect, and the mechanism of which is partially or totally independent from KATP channels. It is hypothesized that nitric oxide donation and free-radical scavenging properties of nicorandil upregulate endothelial nitric oxide synthase may be responsible for this phenomenon. These findings suggest that nicorandil can be useful in treatment of IBD, although further investigations are needed to elucidate the mechanisms involved.
Similar content being viewed by others
References
Jurjus A.R., Khoury N.N., Reimund J.M., Animal models of inflammatory bowel disease, J. Pharmacol. Toxicol. Method., 2004, 50, 81–92
Rezaie A., Parker R.D., Abdollahi M., Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause?, Dig. Dis. Sci., 2007, 52, 2015–2021
Blokhina O., Virolainen E., Fagerstedt K.V., Anti-oxidants, oxidative damage and oxygen deprivation stress: a review, Ann. Bot. (Lond)., 2003, 91, 179–194
Middleton S.J., Shorthouse M., Hunter J.O., Increased nitric oxide synthesis in ulcerative colitis, Lancet, 1993, 341, 465–466
Panaccione R., Ferraz J.G., Beck P., Advances in medical therapy of inflammatory bowel disease, Curr. Opin. Pharmacol., 2005, 5, 566–572
Langmead L., Rampton D.S., Review article: complementary and alternative therapies for inflammatory bowel disease, Review, Aliment. Pharmacol. Ther., 2006, 23, 341–349
Ashtaral-Nakhai L., Mohammadirad A., Yasa N., Minaie B., Nikfar S., Ghazanfari G., et al., Benefits of Zataria multiflora Boiss in experimental model of mouse inflammatory bowel disease, Evid. Based Complement. Alternat. Med., 2007, 4, 43–50
Ghafari H., Yasa N., Mohammadirad A., Dehghan G., Zamani M.J., Nikfar S., et al., Protection by Ziziphora clinopoides of acetic acid-induced toxic bowel inflammation through reduction of cellular lipid peroxidation and myeloperoxidase activity, Hum. Exp. Toxicol., 2006, 25, 325–332
Ghazanfari G., Minaie B., Yasa N., Nakhai L., Mohammadirad A., Nikfar S., et al., Biochemical and histopathological evidences for beneficial effects of Satureja khuzestanica jamzad essential oil on the mouse model of inflammatory bowel diseases, Toxicol. Mech. Methods, 2006, 16, 365–372
Rahimi R., Mozaffari S., Abdollahi M., On the use of herbal medicines in management of inflammatory bowel diseases: a systematic review of animal and human studies, Dig. Dis. Sci., 2008, [Epub ahead of print] (DOI 10.1007/s10620-008-0368-x)
Ebrahimi F., Esmaily H., Baeeri M., Mohammadirad A., Fallah S., Abdollahi M., Molecular evidences on the benefits of N-acetylcysteine in experimental colitis, Cent. Eur. J. Biol., 2008, 3, 135–142
Elahi B., Nikfar S., Derakhshani S., Vafaie M., Abdollahi M., On the benefit of probiotics in the management of pouchitis in patients underwent ileal pouch anal anastomosis: a meta-analysis of controlled clinical trials, Dig. Dis. Sci., 2007, 53, 1278–1284
Rezaie A., Taghavi Bayat B., Abdollahi M., Biologic management of fistulizing Crohn’s disease, Int. J. Pharmacol., 2005, 1, 17–24
Rahimi R., Nikfar S., Rezaie A., Abdollahi M., A meta-analysis of the benefit of probiotics in maintaining remission of human ulcerative colitis: evidence for prevention of disease relapse and maintenance of remission, Arch. Med. Sci., 2008, 4, 185–190
Rahimi R, Nikfar S., Rahimi F., Elahi B., Derakhshani S., Vafaie M., et al., A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn’s disease, Dig. Dis. Sci., 2008, 53, 2524–2531
Rahimi R., Nikfar S., Rezaie A., Abdollahi M., A meta-analysis of antibiotic therapy for active ulcerative colitis, Dig. Dis. Sci., 2007, 52, 2920–2925
Rahimi R., Nikfar S., Rezaie A., Abdollahi M., A meta-analysis of broad spectrum antibiotic therapy in patients with active Crohn’s disease, Clin. Ther., 2006, 28, 1983–1988
Dubinsky M.C., Targeting Therapy in Pediatric Inflammatory Bowel Disease, Curr. Treat. Options Gastroenterol., 2004, 7, 391–405
Akai K., Wang Y., Sato K., Sekiguchi N., Sugimura A., Kumagai T., et al., Vasodilatory effect of nicorandil on coronary arterial microvessels: its dependency on vessel size and the involvement of the ATP-sensitive potassium channels, J. Cardiovasc. Pharmacol., 1995, 26, 541–547
Hosseini-Tabatabaei A., Abdollahi M., Potassium channel openers and improvement of toxic stress: Do they have role in the management of Inflammatory bowel disease?, Inflamm. Allergy Drug Targets, 2008, 7, 129–135
Heywood G.J., Thomas P.S., Nicorandil inhibits degranulation and TNF-alpha release from RBL-2H3 cells, Inflamm. Res., 2002, 51, 176–181
Facundo H.T., De Paula J.G., Kowaltowski A.J., Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production, Free Radic. Biol. Med., 2007, 42, 1039–1048
Facundo H.T., De Paula J.G, Kowaltowski A.J., Mitochondrial ATP-sensitive K+ channels prevent oxidative stress, permeability transition and cell death, J. Bioenerg. Biomembr., 2005, 37, 75–82
Wang Y.P., Maeta H., Mizoguchi K., Suzuki T., Yamashita Y., Oe M., Intestinal ischemia preconditions myocardium: role of protein kinase C and mitochondrial K(ATP) channel, Cardiovasc. Res., 2002, 55, 576–582
Teshima Y., Akao M., Baumgartner W.A., Marbán E., Nicorandil prevents oxidative stress-induced apoptosis in neurons by activating mitochondrial ATP-sensitive potassium channels, Brain. Res., 2003, 990, 45–50
Akao M., Teshima Y., Marbán E., Antiapoptotic effect of nicorandil mediated by mitochondrial atpsensitive potassium channels in cultured cardiac myocytes, J. Am. Coll. Cardiol., 2002, 40, 803–810
Nagata K., Obata K., Odashima M., Yamada A., Somura F., Nishizawa T., et al., Nicorandil inhibits oxidative stress-induced apoptosis in cardiac myocytes through activation of mitochondrial ATP-sensitive potassium channels and a nitrate-like effect, J. Mol. Cell. Cardiol., 2003, 35, 1505–1512
Xu J., Nagata K., Obata K., Ichihara S., Izawa H., Noda A., et al., Nicorandil promotes myocardial capillary and arteriolar growth in the failing heart of Dahl salt-sensitive hypertensive rats, Hypertension, 2005, 46, 719–724
Ismail H.A., Khalifa M.M., Hassan M.K., Ashour O.M., Insights in the mechanisms underlying the anti-ulcer activity of nicorandil, Pharmazie, 2007, 62, 60–67
Mourelle M., Vilaseca J., Guarner F., Salas A., Malagelada J.R., Toxic dilatation of colon in a rat model of colitis is linked to an inducible form of nitric oxide synthase, Am. J. Physiol., 1996, 270, G425–G430
Abdollahi M., Dehpour A.R., Baharnouri G., Alteration by rubidium of rat submandibular secretion of protein and N-acetyl-D-glucosaminidase, Tox. Subst. Mech., 1998, 17, 121–131
Wallace J.L., Keenan C.M., Gale D., Shoupe T.S., Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis, Gastroenterology, 1992, 102, 18–27
Mustafa A., El-Medany A., Hagar H.H., El-Medany G., Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis, Pharmacol. Res., 2006, 53, 324–330
Cuzzocrea S., Ianaro A., Wayman N.S., Mazzon E., Pisano B., Dugo L., et al., The cyclopentenone prostaglandin 15-deoxy-delta(12,14)-PGJ2 attenuates the development of colon injury caused by dinitrobenzene sulphonic acid in the rat, Br. J. Pharmacol., 2003, 138, 678–688
Dehghan G., Shafiee A., Ghahremani M.H., Ardestani S.K., Abdollahi M., Antioxidant potential of various extracts from Ferula szovitsiana in relation to their phenolic content, Pharm. Biol., 2007, 45, 691–699
Astaneie F., Afshari M., Mojtahedi A., Mostafalou S., Zamani M.J., Larijani B., et al., Total antioxidant capacity and levels of epidermal growth factor and nitric oxide in blood and saliva of insulin-dependent diabetic patients, Arch. Med. Res., 2005, 36, 376–381
Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J., Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275
Torres M.I., García-Martin M., Fernández M.I., Nieto N., Gil A., Ríos A., Experimental colitis induced by trinitrobenzenesulfonic acid: an ultrastructural and histochemical study, Dig. Dis. Sci., 1999, 44, 2523–2529
Kolgazi M., Jahovic N., Yuksel M., Ercan F., Alican I., α-lipoic acid modulates gut inflammation induced by trinitrobenzene sulfonic acid in rats, J. Gastroenterol. Hepatol., 2007, 22, 1859–1865
Shen C., De Hertogh G., Bullens D.M., Van Assche G., Geboes K., Rutgeerts P., et al., Remission-inducing effect of anti-TNF monoclonal antibody in TNBS colitis: mechanisms beyond neutralization?, Inflamm. Bowel. Dis., 2007, 13, 308–316
Rahimi R., Nikfar S., Abdollahi M., Do anti-tumor necrosis factors induce response and remission in patients with acute refractory Crohn’s disease? A systematic meta-analysis of controlled clinical trials, Biomed. Pharmacother., 2007, 61, 75–80
Rahimi R., Nikfar S., Abdollahi M., Meta-analysis technique confirms the effectiveness of anti-TNF-a in the management of active ulcerative colitis when administered in combination with corticosteroids, Med. Sci. Monitor., 2007, 13, PI13–PI18
Ludwiczek O., Vannier E., Borggraefe I., Kaser A., Siegmund B., Dinarello C.A., et al., Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease, Clin. Exp. Immunol., 2004, 138, 323–329
Simmonds N.J., Rampton D.S., Inflammatory bowel disease—a radical view, Gut, 1993, 34, 865–868
Suzuki M., Saito T., Sato T., Tamagawa M., Miki T., Seino S., Nakaya H., Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice, Circulation, 2003, 107, 682–685
Hanley P.J., Daut J., K(ATP) channels and preconditioning: a re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms, J. Mol. Cell. Cardiol., 2005, 39, 17–50
Inoue I., Nagase H., Kishi K., Higuti T., ATP-sensitive K+ channel in the mitochondrial inner membrane, Nature, 1991, 352, 244–247
Deby-Dupont G., Deby C., Lamy M., Neutrophil myeloperoxidase revisited: its role in health and disease, Intensivmed., 1999, 36, 500–551
Kruidenier L., Kuiper I., Van Duijn W., Mieremet-Ooms M.A., van Hogezand R.A., Lamers C.B., et al., Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease, J. Pathol., 2003, 201, 17–27
Kruidenier L., Kuiper I., Lamers C.B., Verspaget H.W., Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants, J. Pathol., 2003, 201, 28–36
Ahmed A.O., Sharifzadeh M., Nikfar S., Jamshidi H.R., Abdollahi M., Prevention by L-arginine/nitric oxide of chlordiazepoxide-induced toxic reactions in the rat salivary gland, Toxicol. Mech. Method., 2006, 16, 331–337
Abdollahi M., Dehpour A.R., Shafayee F., L-Arginine/nitric oxide pathway and interaction with lead acetate on rat submandibulary gland function, Pharmacol. Toxicol., 2000, 87, 198–203
Kolios G., Valatas V., Ward S.G., Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle, Immunology, 2004, 113, 427–437
Barrachina M.D., Panes J., Esplugues J.V., Role of nitric oxide in gastrointestinal inflammatory and ulcerative diseases: perspective for drugs development, Curr. Pharm. Des., 2001, 7, 31–48
Jahanshahi G., Motavasel V., Rezaie A., Hashtroudi A.A., Daryani N.E., Abdollahi M., Alterations in antioxidant power and levels of epidermal growth factor and nitric oxide in saliva of patients with inflammatory bowel diseases, Dig. Dis. Sci., 2004, 49, 1752–1757
Horinaka S., Kobayashi N., Yabe A., Asakawa H., Yagi H., Mori Y., et al., Nicorandil protects against lethal ischemic ventricular arrhythmias and up-regulates endothelial nitric oxide synthase expression and sulfonylurea receptor 2 mRNA in conscious rats with acute myocardial infarction, Cardiovasc. Drugs Ther., 2004, 18, 13–22
Horinaka S., Kobayashi N., Higashi T., Hara K., Hara S., Matsuoka H., Nicorandil enhances cardiac endothelial nitric oxide synthase expression via activation of adenosine triphosphate-sensitive K channel in rat, J. Cardiovasc. Pharmacol., 2001, 38, 200–210
Pompermayer K., Souza D.G., Lara G.G., Silveira K.D., Cassali G.D., Andrade A.A., et al., The ATPsensitive potassium channel blocker glibenclamide prevents renal ischemia/reperfusion injury in rats, Kidney Int., 2005, 67, 1785–1796
Pompermayer K., Amaral F.A., Fagundes C.T., Vieira A.T., Cunha F.Q., Teixeira M.M., et al., Effects of the treatment with glibenclamide, an ATP-sensitive potassium channel blocker, on intestinal ischemia and reperfusion injury, Eur. J. Pharmacol., 2007, 556, 215–222
Flavio A.G., Cunha F.Q., Francescato H.D., Soares T.J., Costa R.S., Barbosa Junior F., et al., ATP-sensitive potassium channel blockage attenuates cisplatin-induced renal damage, Kidney Blood Press. Res., 2007, 30, 289–298
Sarkhail P., Rahmanipour S., Fadyevatan S., Mohammadirad A., Dehghan G., Amin G., et al., Antidiabetic effect of Phlomis anisodonta: Effects on hepatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes, Pharmacol. Res., 2007, 56, 261–266
Cocks T.M., King S.J., Angus J.A., Glibenclamide is a competitive antagonist of the thromboxane A2 receptor in dog coronary artery in vitro, Br. J. Pharmacol., 1990, 100, 375–378
Rampton D.S., Collins C.E., Review article: thromboxanes in inflammatory bowel disease—pathogenic and therapeutic implications, Aliment. Pharmacol. Ther., 1993, 7, 357–367
Reichert S., Antunes A., Tréchot P., Barbaud A., Weber M., Schmutz J.L., Major aphthous stomatitis induced by nicorandil, Eur. J. Dermatol., 1997, 7, 132–133
Watson A., Al-Ozairi O., Fraser A., Loudon M., O’Kelly T., Nicorandil associated anal ulceration, Lancet, 2002, 360, 546–547
McKenna D.J., Donnelly J., Armstrong D.K., Nicorandil-induced leg ulceration, Br. J. Dermatol., 2007, 156, 394–396
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hosseini-Tabatabaei, A., Esmaily, H., Rahimian, R. et al. Benefit of nicorandil using an immunologic murine model of experimental colitis. cent.eur.j.biol. 4, 74–85 (2009). https://doi.org/10.2478/s11535-008-0047-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-008-0047-0