Abstract
The phenomenon of circulating cell-free DNA (cfDNA) concentrations is of importance for many biomedical disciplines including the field of exercise physiology. Increases of cfDNA due to exercise are described to be a potential hallmark for the overtraining syndrome and might be related to, or trigger adaptations of, immune function induced by strenuous exercise. At the same time, exercise provides a practicable model for studying the phenomenon of cfDNA that is described to be of pathophysiological relevance for different topics in clinical medicine like autoimmune diseases and cancer.
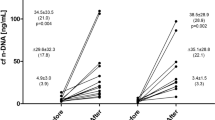
In this review, we are summarizing the current knowledge of exercise-based acute and chronic alterations in cfDNA levels and their physiological significance. The effects of acute exercise on cfDNA concentrations have been investigated in resistance exercises and in continuous, stepwise and interval endurance exercises of different durations. cfDNA concentrations peaked immediately after acute exercise and showed a rapid return to baseline levels. Typical markers of skeletal muscle damage (creatine kinase, uric acid, C-reactive protein) show delayed kinetics compared with the cfDNA peak response. Exercise parameters such as intensity, duration or average energy expenditure do not explain the extent of increasing cfDNA concentrations after strenuous exercise. This could be due to complex processes inside the human organism during and after physical activity. Therefore, we hypothesize composite effects of different physiological stress parameters that come along with exercise to be responsible for increasing cfDNA concentrations. We suggest that due to acute stress, cfDNA levels increase rapidly by a spontaneous active or passive release mechanism that is not yet known. As a result of the rapid and parallel increase of cfDNA and lactate in an incremental treadmill test leading to exhaustion within 15–20 minutes, it is unlikely that cfDNA is released into the plasma by typical necrosis or apoptosis of cells in acute exercise settings. Recently, rapid DNA release mechanisms of activated immune-competent cells like NETosis (pathogen-induced cell death including the release of neutrophil extracellular traps [NETs]) have been discovered. cfDNA accumulations might comprise a similar kind of cell death including trap formation or an active release of cfDNA. Just like chronic diseases, chronic high-intensity resistance training protocols induced persistent increases of cfDNA levels. Chronic, strenuous exercise protocols, either long-duration endurance exercise or regular high-intensity workouts, induce chronic inflammation that might lead to a slow, constant release of DNA. This could be due to mechanisms of cell death like apoptosis or necrosis. Yet, it has neither been implicated nor proven sufficiently whether cfDNA can serve as a marker for overtraining. The relevance of cfDNA with regard to overtraining status, performance level, and the degree of physical exhaustion still remains unclear. Longitudinal studies are required that take into account standardized and controlled exercise, serial blood sampling, and large and homogeneous cohorts of different athletic achievement. Furthermore, it is important to establish standardized laboratory procedures for the measurement of genomic cfDNA concentrations by quantitative real-time polymerase chain reaction (PCR). We introduce a new hypothesis based on acute exercise and chronic exposure to stress, and rapid active and passive chronic release of cfDNA fragments into the circulation.









Similar content being viewed by others
References
Stroun M, Anker P, Lyautey J, et al. Isolation and characterization of DNA from the plasma of cancer patients. Eur J Cancer Clin Oncol 1987 Jun; 23 (6): 707–12
Bennett RM, Gabor GT, Merritt MM. DNA binding to human leukocytes: evidence for a receptor-mediated association, internalization, and degradation of DNA. J Clin Invest 1985 Dec; 76 (6): 2182–90
Tamkovich SN, Cherepanova AV, Kolesnikova EV, et al. Circulating DNA and DNase activity in human blood. Ann N Y Acad Sci 2006 Sep; 1075: 191–6
Anker P, Stroun M, Maurice PA. Spontaneous release of DNA by human blood lymphocytes as shown in an in vitro system. Cancer Res 1975 Sep; 35 (9): 2375–82
Gahan PB. Circulating DNA: intracellular and intraorgan messenger? Ann N Y Acad Sci 2006 Sep; 1075: 21–33
Stroun M, Lyautey J, Lederrey C, et al. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta 2001 Nov; 313 (1–2): 139–42
Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 2001 Feb 15; 61 (4): 1659–65
Suzuki N, Kamataki A, Yamaki J, et al. Characterization of circulating DNA in healthy human plasma. Clinica Chimica Acta 2008; 387: 55–8
Chan KCA, Zhang J, Hui ABY, et al. Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem 2004 Jan; 50 (1): 88–92
Ziegler A, Zangemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treatment Reviews 2002 Oct; 28 (5): 255–71
Van der Vaart M, Pretorius PJ. Circulating DNA: its origin and fluctuation. Ann N Y Acad Sci 2008 Aug; 1137: 18–26
Fehrenbach E, Niess AM, Schlotz E, et al. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol 2000 Aug; 89 (2): 704–10
Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med 2010 Jun; 48 (6): 757–67
Chevion S, Moran DS, Heled Y, et al. Plasma antioxidant status and cell injury after severe physical exercise. Proc Natl Acad Sci U S A 2003 Apr 29; 100 (9): 5119–23
Chan KCA, Lo YMD. Circulating nucleic acids as a tumor marker. Histol Histopathol 2002; 17 (3): 937–43
Melnikov AA, Scholtens D, Talamonti MS, et al. Methylation profile of circulating plasma DNA in patients with pancreatic cancer. J Surg Oncol 2009 Feb 1; 99 (2): 119–22
deVos T, Tetzner R, Model F, et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem 2009 Jul; 55 (7): 1337–46
Gleeson M. Immune function in sport and exercise. J Appl Physiol 2007 Aug; 103 (2): 693–9
Fehrenbach E, Schneider ME. Trauma-induced systemic inflammatory response versus exercise-induced immunomodulatory effects. Sports Med 2006; 36 (5): 373–84
Northoff H, Berg A, Weinstock C. Similarities and differences of the immune response to exercise and trauma: the IFN-gamma concept. Can J Physiol Pharmacol 1998 May; 76 (5): 497–504
Atamaniuk J, Vidotto C, Tschan H, et al. Increased concentrations of cell-free plasma DNA after exhaustive exercise. Clin Chem 2004 Sep; 50 (9): 1668–70
Fatouros IG, Destouni A, Margonis K, et al. Cell-free plasma DNA as a novel marker of aseptic inflammation severity related to exercise overtraining. Clin Chem 2006 Sep; 52 (9): 1820–4
Atamaniuk J, Stuhlmeier KM, Vidotto C, et al. Effects of ultra-marathon on circulating DNA and mRNA expression of pro- and anti-apoptotic genes in mononuclear cells. Eur J Appl Physiol 2008 Nov; 104 (4): 711–7
Atamaniuk J, Vidotto C, Kinzlbauer M, et al. Cell-free plasma DNA and purine nucleotide degradation markers following weightlifting exercise. Eur J Physiol 2010 Nov; 110 (4): 695–701
Fatouros IG, Jamurtas AZ, Nickolaidis MG, et al. Time sampling is crucial for measurements of cell-free plasma DNA following acute aseptic inflammation induced by exercise. Clin Biochem 2010 Nov; 43 (16–17): 1368–70
Beiter T, Fragasso A, Hudemann J, et al. Short-term treadmill running as a model for studying cell-free DNA kinetics in vivo. Clin Chem 2011 Apr; 57 (4): 633–6
Fleischhacker M, Schmidt B, Weickmann S, et al. Methods for isolation of cell-free plasma DNA strongly affect DNA yield. Clin Chim Acta 2011 Nov 20; 412 (23–24): 2085–8
Horlitz M, Lucas A, Sprenger-Haussels M. Optimized quantification of fragmented, free circulating DNA in human blood plasma using a calibrated duplex real-time PCR. PLoS One 2009 Sep 28; 4 (9): e7207
Kiode K, Sekizawa A, Iwasaki M, et al. Fragmentation of cell-free fetal DNA in plasma and urine of pregnant women. Prenat Diagn 2005 Jul; 25 (7): 604–7
Sikora A, Zimmermann BG, Rusterholz C, et al. Detection of increased amounts of cell-free fetal DNA with short PCR amplicons. Clin Chem 2010 Jan; 56 (1): 136–8
Fehrenbach E, Northoff H. Free radicals, exercise, apoptosis, and heat shock proteins. Exerc Immunol Rev 2001; 7: 66–89
Hellsten Y, Hansson HA, Johnson L, et al. Increased expression of xanthine oxidase and insulin-like growth factor I (IGF-I) immunoreactivity in skeletal muscle after strenuous exercise in humans. Acta Physiol Scand 1996 Jun; 157 (2): 191–7
Hellsten Y, Frandsen U, Orthenblad N, et al. Xanthine oxidase in human skeletal muscle following eccentric exercise: a role in inflammation. J Physiol 1997 Jan 1; 498: 239–48
Lo YMD, Zhang J, Leung TN, et al. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 1999 Jan; 64 (1): 218–24
Castiglioni A, Canti V, Rovere-Querini P, et al. Highmobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell Tissue Res 2011; 343: 189–99
Hashimoto T, Hussien R, Oommen S, et al. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J 2007 Aug; 21 (10): 2602–12
Margonis K, Fatouros IG, Jamutas AZ, et al. Oxidative stress biomarkers response to physical overtraining: implications for diagnosis. Free Radic Biol Med 2007 Sep 15; 43 (6): 901–10
Hirose L, Nosaka K, Newton M, et al. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev 2004; 10: 75–90
Coyle EF. Physical activity as a metabolic stressor. Am J Clin Nutr 2000 Aug; 72 (2 Suppl.): 512S–20S
Neubauer O, Reichhold S, Nersesyan A, et al. Exercise-induced DNA damage: is there a relationship with inflammatory responses? Exerc Immunol Rev 2008; 14: 51–72
Lippi G, Schena F, Salvagno GL, et al. Acute variation of biochemical markers of muscle damage following 21-km, half-marathon run. Scand J Clin Lab Invest 2008; 68 (7): 667–72
Kanter MM, Lesmes GR, Kaminsky LA, et al. Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race: relationship to lipid peroxidation. Eur J Appl Physiol Occup Physiol 1988; 57 (1): 60–3
Ratamess NA, Falvo MJ, Mangine GT, et al. The effect of rest interval length on metabolic responses to the bench press exercise. Eur J Appl Physiol 2007 May; 100 (1): 1–17
Lehmann M, Berg A, Kapp R, et al. Correlations between laboratory testing and distance running performance in marathoners of similar performance ability. Int J Sports Med 1983 Nov; 4 (4): 226–30
Niess AM, Fehrenbach E, Strobel G, et al. Evaluation of stress response to interval training at low and moderate altitude. Med Sci Sports Exerc 2003 Feb; 35 (2): 263–9
Williams C, Nute ML. Some physiological demands of a half-marathon race on recreational runners. Br J Sports Med 1983 Sep; 17 (3): 152–61
Zaccaria M, Ermolao A, Roi GS, et al. Leptin reduction after endurance races differing in duration and energy expenditure. Eur J Appl Physiol 2002 Jun; 87 (2): 108–11
Fehrenbach E, Passek F, Niess AM, et al. HSP expression in human leukocytes is modulated by endurance exercise. Med Sci Sports Exerc 2000 Mar; 32 (3): 592–600
Kim HJ, Lee YH, Kim CK. Biomarkers of muscle and cartilage damage and inflammation during 200 km run. Eur J Appl Physiol 2007 Mar; 99 (4): 443–7
Kraemer WJ, Volek JS, Bush JA, et al. Hormonal responses to consecutive days of heavy-resistance exercise with or without nutritional supplementation. J Appl Physiol 1998 Oct; 85(4): 1544–55
Achten J, Venables MC, Jeukendrup AE. Fat oxidation rates are higher during running compared with cycling over a wide range of intensities. Metabolism 2003 Jun; 52 (6):747–52
Niess AM, Hartmann A, Grünert-Fuchs M, et al. DNA damage after exhaustive treadmill running in trained and untrained men. Int J Sports Med 1996 Aug; 17 (6): 397–403
Mastaloudis A, Morrow JD, Hopkins DW, et al. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med 2004 May 15; 36 (10): 1329–41
Noakes TD, Carter JW. The response of plasma biochemical parameters to a 56-km race in novice and experienced ultra-marathon runners. Eur J Appl Physiol. 1982; 42: 179–86
Paul GL, DeLany JP, Snook JT, et al. Serum and urinary markers of skeletal muscle tissue damage after weight lifting exercise. Eur J Appl Physiol Occup Physiol 1989; 58 (7): 786–90
Adams WC, Fox RH, Fry AJ, et al. Thermoregulation during marathon running in cool, moderate, and hot environments. J Appl Physiol 1975 Jun; 38 (6): 1030–7
Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med 2001 Oct 1; 31 (7): 911–22
Dumke CL, Shooter L, Lind RH, et al. Indirect calorimetry during ultradistance running: a case report. J Sci Sports Med 2006; 5: 692–8
Hudson MB, Hosick PA, McCaulley GO, et al. The effect of resistance exercise on humoral markers of oxidative stress. Med Sci Sports Exerc 2008 Mar; 40 (3): 542–8
Scala D, McMillan J, Blessing D, et al. Metabolic cost of a preparatory phase of training in weight lifting: a practical observation. J Appl Sports Sci Res 1987; 1: 48–52
Branth S, Hambraeus L, Piehl-Aulin K, et al., Metabolic stress-like condition can be induced by prolonged strenuous exercise in athletes. Ups J Med Sci 2009; 114(1): 12–25
Cooper CE, Vollaard NB, Choueiri T, et al. Exercise, free radicals and oxidative stress. Biochem Soc Trans 2002 Apr; 30 (2): 280–5
Niess AM, Sommer M, Schlotz E, et al. Expression of the inducible nitric oxide synthase (iNOS) in human leukocytes: responses to running exercise. Med Sci Sports Exerc 2000 Jul; 32 (7): 1220–5
Bianchi DW. Circulating fetal DNA: its origin and diagnostic potential: a review. Placenta 2004 Apr; 25 Suppl. A: 93–101
Mooren FC, Blöming D, Lechtermann A, et al. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol 2002 Jul; 93 (1): 147–53
Goldstein JC, Kluck RM, Green DR. A single cell analysis of apoptosis: ordering the apoptotic phenotype. Ann N Y Acad Sci 2000; 926: 132–41
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972 Aug; 26 (4): 239–57
Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol 1980; 68: 251–306
Goldstein JC, Waterhouse NJ, Juin P, et al. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2000 Mar; 2 (3): 156–62
Mars M, Govender S, Weston A, et al. High intensity exercise: a cause of lymphocyte apoptosis? Biochem Biophys Res Commun 1998 Aug 19; 249 (2): 366–70
Krüger K, Agnischock S, Lechtermann A, et al. Intensive resistance exercise induces lymphocyte apoptosis via cortisol and glucocorticoid receptor-dependent pathways. J Appl Physiol 2011 May; 110(5): 1226–32
Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 2007 Aug; 5 (8): 577–82
Hawley CJ, Schoene RB. Overtraining syndrome: a guide to diagnosis, treatment, and prevention. Phys Sportsmed 2003 Jun; 31 (6): 25–31
Rietjens GJWM, Kuipers H, Adam JJ, et al. Physiological, biochemical and psychological markers of strenuous training-induced fatigue. Int J Sports Med 2005 Jan–Feb; 26 (1): 16–26
Swanson DR. Atrial fibrillation in athletes: implicit literature-based connections suggest that overtraining and subsequent inflammation may be a contributory mechanism. Med Hypotheses 2006; 66 (6): 1085–92
Meeusen R, Watson P, Hasegawa H, et al. Brain neurotransmitters in fatigue and overtraining. Appl Physiol Nutr Metab 2007 Oct; 32 (5): 857–64
Urhausen A, Kindermann W. Diagnosis of overtraining: what tools do we have? Sports Med 2002; 32 (2): 95–102
Urhausen A, Gabriel H, Kindermann W. Blood hormones as markers of training stress and overtraining. Sports Med 1995 Oct; 20 (4): 251–76
Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Bio-markers 2005 Nov; 10 Suppl. 1: 10–23
Halson SL, Jeukendrup AE. Does overtraining exist? An analysis of overreaching and overtraining research. Sports Med 2004; 34 (14): 967–81
Mackinnon LT. Overtraining effects on immunity and performance in athletes. Immun Cell Bio 2000; 78: 502–9
Stroun M, Maurice P, Vasioukhin V, et al. The origin and mechanism of circulating DNA. Ann N Y Acad Sci 2000 Apr; 906: 161–8
Pedersen BK, Steensberg A. Exercise and hypoxia: effects on leukocytes and interleukin-6-shared mechanisms? Med Sci Sports Exerc 2002 Dec; 34 (12): 2004–13
von Köckritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl) 2009 Aug; 87 (8): 775–83
Record M, Subra C, Silvente-Poirot S, et al. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 2011 May 15; 81 (10): 1171–82
Kocsis AK, Szabolcs A, Hofner P, et al. Plasma concentrations of high-mobility group box protein 1, soluble receptor for advanced glycation end-products and circulating DNA in patients with acute pancreatitis. Pancreatology 2009; 9 (4): 383–91
Acknowledgements
The authors thank the reviewer for the many constructive comments on the first version of their review and Katherine E. Curtis for proof reading the manuscript. No funding was received to assist in the preparation of this article. The authors have no conflicts of interest to declare that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breitbach, S., Tug, S. & Simon, P. Circulating Cell-Free DNA. Sports Med 42, 565–586 (2012). https://doi.org/10.2165/11631380-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11631380-000000000-00000