Abstract
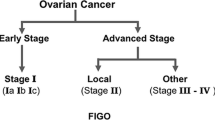
Although ovarian cancer is often a chemosensitive malignancy, patients who are resistant to platinum-based chemotherapy represent a therapeutic challenge. Currently, the only drugs that are US FDA approved to treat this subset of patients are paclitaxel, pegylated liposomal doxorubicin (PLD) and topotecan. The response rates with these agents is in the 10–15% range and overall survival is around 12 months. Other drugs that have shown some activity in platinum-resistant ovarian cancer include the taxane analogues, oral etoposide, pemetrexed and bevacizumab. Unfortunately, randomized phase III trials of second-line chemotherapy in patients with platinum-resistant ovarian cancer have not shown an advantage over existing therapy with respect to progression-free survival or overall survival. The only trial that has reported a significant progression-free survival advantage over standard therapy is a randomized phase II trial of PLD with or without EC145, a folate-linked vinca alkaloid. Final survival results of this trial are pending.





Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010 Sep–Oct; 60(5): 277–300
Markman M, Rothman R, Hakes T, et al. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991 Mar; 9(3): 389–93
Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006 May 1; 106(9): 1933–9
Harter P, du Bois A, Hahmann M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol 2006 Dec; 13(12): 1702–10
McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Int Med 1989 Aug 15; 111(4): 273–9
Thigpen JT, Blessing JA, Ball H, et al. Phase II trial of paclitaxel in patients with progressive ovarian carcinoma after platinum-based chemotherapy: a Gynecologic Oncology Group study. J Clin Oncol 1994 Sep; 12(9): 1748–53
Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med 1995 Apr 13; 332(15): 1004–14
McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophos-phamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996 Jan 4; 334(1): 1–6
Markman M, Blessing J, Rubin SC, et al. Phase II trial of weekly paclitaxel (80mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol 2006 Jun; 101(3): 436–40
Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009 Oct 17; 374(9698): 1331–8
Rosenberg P, Andersson H, Boman K, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol 2002; 41(5): 418–24
ten Bokkel Huinink W, Gore M, Carmichael J, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 1997 Jun; 15(6): 2183–93
ten Bokkel Huinink W, Lane SR, Ross GA. Long-term survival in a phase III, randomised study of topotecan versus paclitaxel in advanced epithelial ovarian carcinoma. Ann Oncol 2004 Jan; 15(1): 100–3
Gore M, ten Bokkel Huinink W, Carmichael J, et al. Clinical evidence for topotecan-paclitaxel non-cross-resistance in ovarian cancer. J Clin Oncol 2001 Apr 1; 19(7): 1893–900
Frykman G, Williams G, Pazdur R. Conflicting phase II efficacy data for Doxil. J Clin Oncol 2001 Jan 15; 19(2): 596–7
Gordon AN, Granai CO, Rose PG, et al. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol 2000 Sep; 18(17): 3093–100
Muggia FM, Hainsworth JD, Jeffers S, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol 1997 Mar; 15(3): 987–93
Gordon AN, Tonda M, Sun S, et al. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol 2004 Oct; 95(1): 1–8
Gordon AN, Fleagle JT, Guthrie D, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 2001 Jul 15; 19(14): 3312–22
Rose PG, Blessing JA, Mayer AR, et al. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 1998 Feb; 16(2): 405–10
Manetta A, Blessing JA, Hurteau JA. Evaluation of cisplatin and cyclosporin A in recurrent platinum-resistant ovarian cancer: a phase II study of the gynecologic oncology group. Gynecol Oncol 1998 Jan; 68(1): 45–6
Markman M, Blessing JA, Moore D, et al. Altretamine (hexamethylmelamine) in platinum-resistant and platinum-refractory ovarian cancer: a Gynecologic Oncology Group phase II trial. Gynecol Oncol 1998 Jun; 69(3): 226–9
Plaxe SC, Blessing JA, Morgan MA, et al. Phase II trial of pyrazoloacridine in recurrent platinum-resistant ovarian cancer: a Gynecologic Oncology Group study. Am J Clin Oncol 2002 Feb; 25(1): 45–7
Fracasso PM, Brady MF, Moore DH, et al. Phase II study of paclitaxel and valspodar (PSC 833) in refractory ovarian carcinoma: a gynecologic oncology group study. J Clin Oncol 2001 Jun 15; 19(12): 2975–82
Hoffman MA, Blessing JA, Morgan M. Phase II trial of CI-958 in recurrent platinum-refractory ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2000 Dec; 79(3): 463–5
Markman M, Blessing JA, DeGeest K, et al. Lack of efficacy of 24-h infusional topotecan in platinum-refractory ovarian cancer: a Gynecologic Oncology Group trial. Gynecol Oncol 1999 Dec; 75(3): 444–6
Miller DS, Blessing JA, Waggoner S, et al. Phase II evaluation of 9-aminocamptothecin (9-AC, NSC #603071) in platinum-resistant ovarian and primary peritoneal carcinoma: a Gynecologic Oncology Group Study. Gynecol Oncol 2005 Jan; 96(1): 67–71
Rose PG, Blessing JA, Ball HG, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2003 Feb; 88(2): 130–5
Fracasso PM, Blessing JA, Morgan MA, et al. Phase II study of oxaliplatin in platinum-resistant and refractory ovarian cancer: a gynecologic group study. J Clin Oncol 2003 Aug 1; 21(15): 2856–9
Brewer CA, Blessing JA, Nagourney RA, et al. Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol 2006 Nov; 103(2): 446–50
De Geest K, Blessing JA, Morris RT, et al. Phase II clinical trial of ixabepilone in patients with recurrent or persistent platinum- and taxane-resistant ovarian or primary peritoneal cancer: a gynecologic oncology group study. J Clin Oncol Jan 1; 28 (1): 149–53
Miller DS, Blessing JA, Krasner CN, et al. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol 2009 Jun 1; 27(16): 2686–91
Coleman RL, Brady WE, McMeekin DS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: A Gynecologic Oncology Group Study. Gynecol Oncol 2011 Jul; 122(1): 111–5
Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Nat Cancer Inst 2004 Nov 17; 96(22): 1682–91
Tinker AV, Gebski V, Fitzharris B, et al. Phase II trial of weekly docetaxel for patients with relapsed ovarian cancer who have previously received paclitaxel: ANZGOG 02-01. Gynecol Oncol 2007 Mar; 104(3): 647–53
Berkenblit A, Seiden MV, Matulonis UA, et al. A phase II trial of weekly docetaxel in patients with platinum-resistant epithelial ovarian, primary peritoneal serous cancer, or fallopian tube cancer. Gynecol Oncol 2004 Dec; 95(3): 624–31
Swensen RE, Childs J, Higgins D, et al. A phase II trial of weekly nab-paclitaxel with GM-CSF as chemoimmunotherapy for platinum-resistant epithelial ovarian cancer [abstract no. 5020]. J Clin Oncol 2011 Jun 9; 29 (15 Suppl.)
Mendelsohn LG, Shih C, Chen VJ, et al. Enzyme inhibition, polyglutamation, and the effect of LY231514 (MTA) on purine biosynthesis. Sem Oncol 1999 Apr; 26 (2 Suppl. 6): 42–7
Miotti S, Bagnoli M, Ottone F, et al. Simultaneous activity of two different mechanisms of folate transport in ovarian carcinoma cell lines. J Cell Biochem 1997 Jun 15; 65(4): 479–91
Hurteau JA, Blessing JA, DeCesare SL, et al. Evaluation of recombinant human interleukin-12 in patients with recurrent or refractory ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2001 Jul; 82(1): 7–10
Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohisto-chemical expression: a Gynecologic Oncology Group study. Clin Cancer Res 2005 Aug 1; 11(15): 5539–48
Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2007 Nov 20; 25(33): 5165–71
Schilder RJ, Sill MW, Lee RB, et al. Phase II evaluation of imatinib mesylate in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 2008 Jul 10; 26(20): 3418–25
Matei D, Sill M, DeGeest K, et al. Phase II trial of sorafenib in persistent or recurrent epithelial ovarian cancer (EOC) or primary peritoneal cancer (PPC): a Gynecologic Oncology Group (GOG) study [abstract no. 5537]. J Clin Oncol 2008; 26 (15 Suppl.)
Modesitt SC, Sill M, Hoffman JS, et al. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2008 May; 109(2): 182–6
Behbakht K, Sill M, Darcy KM, et al. Phase II trial of the mTOR inhibitor temsirolimus and evaluation of circulating tumor cells in persistent or recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study [abstract no. 7]. Gynecol Oncol 2010; 116 (3)
Usha L, Sill M, Darcy KM, et al. A Gynecologic Oncology Group phase II trial of the protein kinase C-beta inhibitor enzastaurin and evaluation of markers with potential predicitive and prognostic value in persistent or recurrent epithelial ovarian and promary peritoneal malignancies [abstract 37]. Gynecol Oncol 2010; 116 Suppl. 1
Rocereto TF, Brady WE, Shahin MS, et al. A phase II evaluation of mifepristone in the treatment of recurrent or persistent epithelial ovarian, fallopian or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2010 Mar; 116(3): 332–4
Sabbatini P, Sill MW, O’Malley D, et al. A phase II trial of paclitaxel poliglumex in recurrent or persistent ovarian or primary peritoneal cancer (EOC): a Gynecologic Oncology Group Study. Gynecol Oncol 2008 Dec; 111(3): 455–60
Kavanagh JJ, Sill MW, Ramirez PT, et al. Phase II multi-center open-label study of karenitecin in previously treated epithelial ovarian and primary peritoneal cancer: a Gynecologic Oncology Group Study. Int J Gynecol Cancer 2008 May–Jun; 18(3): 460–4
Monk BJ, Sill MW, Hanjani P, et al. Activity of docetaxel plus trabectedin in recurrent or persistent ovarian and primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group [abstract no. 5046]. J Clin Oncol 2010; 28 (15 Suppl.)
Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol 2009 Mar 20; 27(9): 1419–25
Bolis G, Parazzini F, Scarfone G, et al. Paclitaxel vs epidoxorubicin plus paclitaxel as second-line therapy for platinum-refractory and -resistant ovarian cancer. Gynecol Oncol 1999 Jan; 72(1): 60–4
Piccart MJ, Green JA, Lacave AJ, et al. Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: a randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol 2000 Mar; 18(6): 1193–202
Buda A, Floriani I, Rossi R, et al. Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group. Br J Cancer 2004 Jun 1; 90(11): 2112–7
Mutch DG, Orlando M, Goss T, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol 2007 Jul 1; 25(19): 2811–8
Ferrandina G, Ludovisi M, Lorusso D, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol 2008 Feb 20; 26(6): 890–6
Meier W, du Bois A, Reuss A, et al. Topotecan versus treosulfan, an alkylating agent, in patients with epithelial ovarian cancer and relapse within 12 months following 1st-line platinum/paclitaxel chemotherapy: a prospectively randomized phase III trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 2009 Aug; 114(2): 199–205
Vergote I, Finkler N, del Campo J, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third-line therapy in patients with platinum-refractory or -resistant ovarian cancer. Eur J Cancer 2009 Sep; 45(13): 2324–32
Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol 2010 Jul 1; 28(19): 3107–14
Naumann RW, Symanowski JT, Ghamande SA, et al. PRECEDENT: a randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum-resistant ovarian cancer [abstract no. LBA5012b]. J Clin Oncol 2010; 28 (18 Suppl.)
Colombo N, Schwartz PE, Bamias A, et al. Results of a randomized, open-label, phase III trial of patupilone (P) versus pegylated liposomal doxorubicin (PLD) in taxane/platinum refractory/resistant patients with recurrent ovarian, fallopian or peritoneal cancer. Ann Oncol 2010; 21 Suppl. 8
Kavanagh JJ, Gershenson DM, Choi H, et al. Multi-institutional phase 2 study of TLK286 (TELCYTA, a glutathione S-transferase P1-1 activated glutathione analog prodrug) in patients with platinum and paclitaxel refractory or resistant ovarian cancer. Int J Gynecol Cancer 2005 Jul–Aug; 15(4): 593–600
Monk B, Herzog T, Kaye S, et al. Final survival results of the randomized phase II study of trabectedin with pegylated liposomal doxorubicin versus pegylated liposomal doxorubicin in recurrent ovarian cancer [abstract no. 5046]. J Clin Oncol 2011; 29 Suppl.
Naumann RW, Coleman RL, Burger RA, et al. PRECEDENT: a randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum-resistant ovarian cancer. J Clin Oncol 2011 Jun 9; 29 (15 Suppl.): 5045
Jurado JM, Sanchez A, Pajares B, et al. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol 2008 Sep; 10(9): 583–6
Smerdel MP, Steffensen KD, Waldstrom M, et al. The predictive value of serum VEGF in multiresistant ovarian cancer patients treated with bevacizumab. Gynecol Oncol 2010 Aug 1; 118(2): 167–71
Tillmanns TD, Lowe MP, Schwartzberg MS, et al. A phase II study of bevacizumab with nab-paclitaxel in patients with reucrrent, platinum-resistant primary epithelial ovarian or peritoneal carcinoma [abstract no. 5009]. J Clin Oncol 2010; 28 (15 Suppl.)
Hoffmann-La Roche. AURELIA: A study of avastin (bevacizumab) added to chemotherapy in patients with platinum-resistant ovarian cancer [ClinicalTrials.gov identifier NCT00976911]. US National Institutes of Health, Clinical-Trials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00976911 [Accessed 2011 Jun 28]
Karlan BY, Oza AM, Hansen L, et al. Randomized, double-blind, placebo-controlled phase II study of AMG 386 combined with weekly paclitaxel in patients with recurrent ovarian carcinoma [abstract no. 5000]. J Clin Oncol 2010; 28 (15 Suppl.)
Coleman RL, Duska LR, Ramirez PT, et al. Phase II multi-institutional study of docetaxel plus aflibercept (AVE0005, NSC# 724770) in patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer [abstract]. J Clin Oncol 2011 Jun 9; 29 (15 Suppl.): 5017
Matei D, Shen C, Fang F, et al. A phase II study of decitabine and carboplatin in recurrent platinum (Pt)-resistant ovarian cancer (OC) [abstract]. J Clin Oncol 2011 Jun 9; 29 (15 Suppl.): 5011
Armstrong DK, Bicher A, Coleman RL, et al. Exploratory phase II efficacy study of MORAb-003, a monoclonal antibody against folate receptor alpha, in platinum-sensitive ovarian cancer [abstract no. 5500]. J Clin Oncol 2008; 26 (15 Suppl.)
Markman M, Markman J, Webster K, et al. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J Clin Oncol 2004; 22(15): 3120–5
Spannuth WA, Lyn YG, Merritt WM, et al. Therapeutic efficacy of folate receptor alpha blockade with MORAb-003 in ovarian cancer. Gynecol Oncol 2008; 108 (3 Suppl. 1): S135
Elit L, Konner JA, Armstrong DK, et al. A randomized, double-blind placebo-controlled phase III study of the efficacy and safety of arletuzumab (MORab-003) incombinatin with weekly paclitaxel in subjects with platinum-resistnat or refractory relapsed ovarian cancer [abstract no. TPS255]. J Clin Oncol 2010; 28 (15 Suppl.)
Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 2010 May 20; 28(15): 2512–9
Birrer MJ, Konstantinopoulos P, Penson RT, et al. A phase II trial of iniparib (BSI-201) in combination with gemcitabine/carboplatin (GC) in patients with platinum-resistant recurrent ovarian cancer [abstract]. J Clin Oncol 2011 Jun 9; 29 (15 Suppl.): 5005
Nishimura S, Tsuda H, Hashiguchi Y, et al. Phase II study of irinotecan plus doxorubicin for early recurrent or platinum-refractory ovarian cancer: interim analysis. Int J Gynecol Cancer 2007 Jan–Feb; 17(1): 159–63
Germano D, Rosati G, Manzione L. Gemcitabine combined with oxaliplatin (GEMOX) as salvage treatment in elderly patients with advanced ovarian cancer refractory or resistant to platinum: a single institution experience. J Chemother 2007 Oct; 19(5): 577–81
Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer [published erratum appears in J Clin Oncol 2008 Apr 1; 26 (10): 1773]. J Clin Oncol 2007 Nov 20; 25(33): 5180–6
Strauss HG, Hemsen A, Karbe I, et al. Phase II trial of biweekly pegylated liposomal doxorubicin in recurrent platinum-refractory ovarian and peritoneal cancer. Antic-ancer Drugs 2008 Jun; 19(5): 541–5
Ray-Coquard I, Weber B, Cretin J, et al. Gemcitabine-oxaliplatin combination for ovarian cancer resistant to taxane-platinum treatment: a phase II study from the GINECO group. Br J Cancer 2009 Feb 24; 100(4): 601–7
Gupta D, Owers RL, Kim M, et al. A phase II study of weekly topotecan and docetaxel in heavily treated patients with recurrent uterine and ovarian cancers. Gynecol Oncol 2009 Jun; 113(3): 327–30
Itani Y, Hosokawa K, Ito K, et al. A phase I/II study of docetaxel and gemcitabine combination for chemotherapy-resistant ovarian cancer. Anticancer Res 2009 May; 29(5): 1521–6
Pectasides D, Pectasides E, Papaxoinis G, et al. Carboplatin/gemcitabine alternating with carboplatin/pegylated liposomal doxorubicin and carboplatin/cyclophosphamide in platinum-refractory/resistant paclitaxel-pretreated ovarian carcinoma. Gynecol Oncol 2010 Jul; 118(1): 52–7
Vergote IB, Micha JP, Pippitt CH, et al. Phase II study of NKTR-102 in women with platinum-resistant/refractory ovarian cancer [abstract]. J Clin Oncol 2010; 28 (15 Suppl.): 5012
Food and Drug Administration. NDA 22-447 ODAC briefing document: Yondelis (trabectidin) [online]. Available from URL: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM171149.pdf [Accessed 2011 Jun 13]
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009 Jan; 45(2): 228–47
Bast RC, Thigpen JT, Arbuck SG, et al. Clinical trial end-points in ovarian cancer: report of an FDA/ASCO/AACR Public Workshop. Gynecol Oncol 2007 Nov; 107(2): 173–6
Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 2000 Jan; 18(1): 106–15
Acknowledgements
Dr Naumann has provided consulting services (without honorarium) to GlaxoSmithKline (GSK), Endocyte, OSI, Novartis, Abraxis, Merck and the GOG. He is involved in research that provides institutional support (without direct payment) sponsored by Amgen, Boehringer Ingelheim, Endocyte, OSI, Novartis, Morphotex and the GOG. He is the Principle Investigator for two phase II trials of EC145 (Endocyte).
In the last year, Professor Coleman has provided consulting, advisory board or speaking services to Abbott Laboratories, Allos Therapeutics, BiPar, Boehringer Ingelheim, Eli Lily, Endocyte, GSK, Sanofi-Aventis, Amgen, AstraZeneca, Centocor, Daiichi Sankyo, Genentech, Merck, Nektar and Ortho Biotech; and received research funding from Novartis, Abraxis, Sanofi-Aventis and Morphotek. None of the relationships listed has influenced the content of the article but are listed for full disclosure purposes. Professor Coleman is the Ann Rife Cox Professor in Gynecology; portions of this work were additionally supported by the Ovarian Cancer Research Fund Inc. (Program Project Development Grant) and the National Institutes of Health (P50 CA098258).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naumann, R.W., Coleman, R.L. Management Strategies for Recurrent Platinum-Resistant Ovarian Cancer. Drugs 71, 1397–1412 (2011). https://doi.org/10.2165/11591720-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11591720-000000000-00000