Abstract
Background: Marketing authorization for rimonabant was withdrawn in October 2008, mainly because the psychiatric adverse effects could not be addressed by further risk minimization.
Objective: The aim of the study was to compare the risk of major and minor depressive episodes in the 6 months prior to and the 6 months after starting treatment with rimonabant.
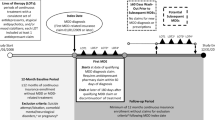
Methods: We conducted a before and after study using the observational cohort technique of Modified Prescription Event Monitoring to compare the risk of major and minor depressive episodes in new users of rimonabant reported in the 6 months before to the 6 months after starting treatment with rimonabant. Patients were identified from dispensed prescriptions issued by primary care physicians from June 2006 to October 2008. Patient demographics and information on depressive episodes were requested 6 months after the date of the first prescription for each patient. Risk ratios (RR) were calculated by comparing before and after events using a matched analysis.
Results: The cohort comprised 10011 patients. The number of patients who had major depressive episodes before and after starting treatment were 147 and 168, respectively (RR 1.14; 95% CI 0.94, 1.39) and the number of patients who had minor depressive episodes were 825 and 829, respectively (RR 1.00; 95% CI 0.93, 1.10). For patients who had a previous history of psychiatric illness (n= 1132), 91 and 73, respectively, experienced major depressive episodes (RR 0.80; 95% CI 0.62,1.03), and 367 and 220, respectively, experienced minor depressive episodes (RR 0.59; 95% CI 0.53, 0.68). For patients without a previous history of psychiatric illness (n = 8879), 56 and 95, respectively, experienced major depressive episodes (RR 1.7; 95% CI 1.2,2.3), and 458 and 609, respectively, experienced minor depressive episodes (RR 1.33; 95% CI 1.20, 1.48).
Conclusions: When comparing all patients in the cohort, there was no increased risk of developing a depressive episode whilst taking rimonabant. However, when considering subsets of patients with and without a previous history of psychiatric illness, the risk profiles were different. In patients without a previous history of psychiatric illness, there were more depressive episodes in the 6 months after starting treatment compared with the 6 months before starting treatment with rimonabant.



Similar content being viewed by others
References
Sanofi-aventis. Acomplia: summary of product characteristics [online]. Available from URL: http://www.acompliareport.com/AcompliaSPC.htm [Accessed 2011 Feb 18]
Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol 2003; 63(4): 908–14
Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004; 3(9): 771–84
Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol 2008; 13(2): 196–212
World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization, 2000
Rennie KL, Jebb SA. Prevalence of obesity in Great Britain. Obes Rev 2005; 6(1): 11–2
Bergstrom A, Pisani P, Tenet V, et al. Overweight as an avoidable cause of cancer in Europe. Int J Cancer 2001; 91(3): 421–30
Ducimetiere P, Richard J, Cambien F. The pattern of subcutaneous fat distribution in middle-aged men and the risk of coronary heart disease: the Paris prospective study. Int J Obes 1986; 10(3): 229–40
Dyer AR, Elliott P. The INTERSALT study: relations of body mass index to blood pressure. INTERSALT Co-Operative Research Group. J Hum Hypertens 1989; 3(5): 299–308
Larsson B, Svardsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13-year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984; 288(6428): 1401–4
Ohlson LO, Larsson B, Svardsudd K, et al. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985; 34(10): 1055–8
Kivimaki M, Lawlor DA, Singh-Manoux A, et al. Common mental disorder and obesity: insight from four repeat measures over 19 years: prospective Whitehall II cohort study. BMJ 2009; 339: b3765
Kivimaki M, Batty GD, Singh-Manoux A, et al. Association between common mental disorder and obesity over the adult life course. Br J Psychiatry 2009; 195(2): 149–55
Sarwer DB, Cohn NI, Gibbons LM, et al. Psychiatric diagnoses and psychiatric treatment among bariatric surgery candidates. Obes Surg 2004; 14(9): 1148–56
Wadden TA, Sarwer DB, Womble LG, et al. Psychosocial aspects of obesity and obesity surgery. Surg Clin North Am 2001; 81(5): 1001–24
Roberts RE, Kaplan GA, Shema SJ, et al. Are the obese at greater risk for depression? Am J Epidemiol 2000; 152(2): 163–70
Roberts RE, Deleger S, Strawbridge WJ, et al. Prospective association between obesity and depression: evidence from the Alameda County study. Int J Obes Relat Metab Disord 2003; 27(4): 514–21
Ross CE. Overweight and depression. J Health Soc Behav 1994; 35(1): 63–79
European Medicines Agency. European Medicines Agency recommends Acomplia must not be used in patients on antidepressants or with major depression [media release]. 2007 Jul 19 [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500012384.pdf [Accessed 2011 Apr 19]
European Medicines Agency. The European Medicines Agency recommends suspension of the marketing authorisation of Acomplia [media release]. 2008 Oct 23 [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500014774.pdf [Accessed 2011 Apr 19]
Van Gaal LF, Rissanen AM, Scheen AJ, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005; 365(9468): 1389–97
Després JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005; 353(20): 2121–34
Christensen R, Kristensen PK, Bartels EM, et al. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007; 370(9600): 1706–13
Shakir SAW. Prescription-event monitoring. In: Mann RD, Andrews EB, editors. Pharmacovigilance. Chichester: John Wiley & Sons Ltd, 2007: 307–16
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Arlington (VA): American Psychiatric Association, 2005
CIOMS-WHO. International guidelines for biomedical research involving human subjects. Geneva: CIOMS-WHO, 2002
Greenland S. Applications of stratified analysis methods. In: Rothman K, Greenland S, editors. Modern epidemiology. 2nd ed. Philadelphia (PA); Lippincott Williams and Wilkins, 2007: 281–99
Cummings P, McKnight B. Analysis of matched cohort data. Stata J 2006; 4(3): 274–81
Medicines and Healthcare products Regulatory Agency. Rimonabant: depression; psychiatric reactions. Drug Saf Update 2008; 5(10): 2–4
European Medicines Agency. Questions and answers on the recommendation to suspend the marketing authorisation of Acomplia (rimonabant) [media release]. 2008 Oct 23 [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/11/WC500014779.pdf [Accessed 2011 Apr 19]
McAvoy BR, Kaner EF. General practice postal surveys: a questionnaire too far? BMJ 1996; 313(7059): 732–3
Office of National Statistics. Migration statistics 2008: annual report [online]. Available from URL: http://www.statistics.gov.uk/downloads/theme_population/Migration-Statistics-2008-Annual-Report.pdf [Accessed 2010 Feb 17]
Acknowledgements
The DSRU is an independent charity (No. 327206) and is an Associate Department of the School of Pharmacy and Biomedical Sciences, University of Portsmouth. It receives unconditional donations from pharmaceutical companies. The companies have no control on the conduct or publication of the studies conducted by the DSRU. The DSRU has received such funds from the manufacturer of rimonabant. Victoria Cornelius and Saad Shakir have received consultancy fees for work unrelated to this project. The other authors have no potential conflicts of interest to declare that are directly relevant to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buggy, Y., Cornelius, V., Wilton, L. et al. Risk of Depressive Episodes with Rimonabant. Drug-Safety 34, 501–509 (2011). https://doi.org/10.2165/11588510-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11588510-000000000-00000