Abstract
Background: The limited use of nortriptyline for smoking cessation is likely due to concerns about its serious adverse effects.
Objective: To examine the safety of nortriptyline at doses equivalent to those used in aiding smoking cessation.
Data Sources: A systematic search of relevant articles in MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews, CINAHL, PsychINFO, WHO publications and the Clinical Trials database (through November 2008).
Study Selection: All studies of nortriptyline at doses between 75 and 100 mg in any indication were reviewed.
Data Extraction: The quality of included studies was assessed based on the Jadad score. Data were extracted using a data extraction form.
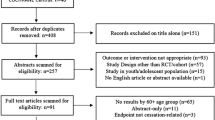
Data Synthesis: From 442 potentially relevant articles identified, 17 studies met our selection criteria and were included for data analysis. Indications for nortriptyline in these studies were smoking cessation (eight studies), depression (five studies), neuropathic pain (three studies) and schizophrenia (one study). 2885 individuals participated in these studies, with exposure time ranging between 4 and 12 weeks. The major comparator used in these trials was placebo. Overall, no life-threatening events occurred in these studies. Orthostatic hypotension was significantly higher in nortriptyline users than in comparator groups (relative risk 2.8; 95% CI 1.4, 5.3). Other adverse events significantly associated with nortriptyline were anticholinergic-related effects including drowsiness, dizziness, gastrointestinal disturbance and dysgeusia.
Conclusions: Current evidence suggests that nortriptyline, at doses between 75 and 100 mg, is not significantly associated with serious adverse events when administered in patients without underlying cardiovascular disease.








Similar content being viewed by others
References
Ezzati M, Lopez AD, Rodgers A, et al. Selected major risk factors and global and regional burden of disease. Lancet 2002; 360: 1347–60
World Health Organization. WHO report on the global tobacco epidemic: the MPOWER package 2008 [online]. Available from URL: http://www.who.int/ [Accessed 2009 Jun 23]
Rasmussen SR, Prescott E, Sorensen TI, et al. The total lifetime health cost savings of smoking cessation to society. Eur J Public Health 2005; 15: 601–6
Quist-Paulsen P, Lydersen S, Bakke PS, et al. Cost effectiveness of a smoking cessation program in patients admitted for coronary heart disease. Eur J Cardiovasc Prev Rehabil 2006; 13: 274–80
Kahn R, Robertson RM, Smith R, et al. The impact of prevention on reducing the burden of cardiovascular disease. Circulation 2008; 118: 576–85
Giovino GA, Shelton DM, Sehooley MW. Trends in cigarette smoking cessation in the United States. Tob Control 1993;2 Suppl.:S3-10
Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. JAMA 2002; 288: 1260–4
Hatsukami DK, Stead LF, Gupta PC. Tobacco addiction. Lancet 2008; 371: 2027–38
Raw M, Anderson P, Batra A, et al. WHO Europe evidence based recommendations on the treatment of tobacco dependence. Tob Control 2002; 11: 44–6
West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Thorax 2000; 55: 987–99
Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 2008; 35: 158–76
Hughes JR, Stread LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007; (1): CD000031
Hughes JR, Stead LF, Lancaster T. Nortriptyline for smoking cessation: a review. Nicotine Tob Res 2005; 7: 491–9
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 464–8
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88
Prochazka AV, Weaver MJ, Keller RT, et al. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med 1998; 158: 2035–9
Hall SM, Humfleet GL, Reus VI, et al. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry 2002; 59: 930–6
da Costa CL, Younes RN, Lourenco MT. Stopping smoking: a prospective, randomized, double-blind study comparing nortriptyline to placebo. Chest 2002; 122: 403–8
Hall SM, Humfleet GL, Reus VI, et al. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry 2004; 161: 2100–7
Prochazka AV, Kick S, Steinbrunn C, et al. A randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation. Arch Intern Med 2004; 164: 2229–33
Wagena EJ, Knipschild PG, Huibers MJ, et al. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med 2005; 165: 2286–92
Haggstram FM, Chatkin JM, Sussenbach-Vaz E, et al. A controlled trial of nortriptyline, sustained-release bupropion and placebo for smoking cessation: preliminary results. Pulm Pharmacol Ther 2006; 19: 205–9
Aveyard P, Johnson C, Fillingham S, et al. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ 2008; 336: 1223–7
Georgotas A, McCue RE, Friedman E, et al. A placebo-controlled comparison of the effect of nortriptyline and phenelzine on orthostatic hypotension in elderly depressed patients. J Clin Psychopharmacol 1987; 7: 413–6
Fabre LF, Scharf MB, Itil TM. Comparative efficacy and safety of nortriptyline and fluoxetine in the treatment of major depression: a clinical study. J Clin Psychiatry 1991; 52 Suppl.: S62-7
Nair NP, Amin M, Holm P, et al. Moclobemide and nortriptyline in elderly depressed patients: a randomized, multicentre trial against placebo. J Affect Disord 1995; 33: 1–9
Bondareff W, Alpert M, Friedhoff AJ, et al. Comparison of sertraline and nortriptyline in the treatment of major depressive 210 disorder in late life. Am J Psychiatry 2000; 157: 729–36
Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus floxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry 2000; 157: 351–9
Haider I. A comparative trial of nortriptyline and ami-triptyline in chronic schizophrenia. Br J Clin Pract 1966; 20: 416–7
Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 2002; 98: 195–203
Chandra K, Shafiq N, Pandhi P, et al. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial. The GONIP Trial. Int J Clin Pharmacol Ther 2006; 44: 358–63
Khoromi S, Cui L, Nackers L, et al. Morphine, nortriptyline and their combination vs placebo in patients with chronic lumbar root pain. Pain 2007; 130: 66–75
Wagena EJ, Knipschild P, Zeegers MPA. Should nortriptyline be used as a first-line aid to help smokers quit? Results from a systematic review and meta-analysis. Addiction 2005; 100: 317–26
Gasto C, Navarro V, Marcos T, et al. Single-blind comparison of venlafaxine and nortriptyline in elderly major depression. J Clin Psychopharmacol 2003; 23: 21–6
Raisi F, Habibi N, Nasehi AA, et al. Combination of citalopram and nortriptyline in the treatment of severe major depression: a double-blind, placebo-controlled trial. Iran J Psychiatry 2006; 1: 35–8
Baldessarini RJ. Drug therapy of depression and anxiety disorders. In: Brunton LL, editor. Goodman & Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006: 429–59
Gibson CA, Bailey BW, Carper MJ, et al. Author contacts for retrieval of data for a meta-analysis on exercise and diet restriction. Int J Technol Assess Health Care 2006; 22: 267–70
Otsubo T, Akimoto Y, Yamada H, et al. A comparative study of the efficacy and safety profiles between fluvox-amine and nortriptyline in Japanese patients with major depression. Pharmacopsychiatry 2005; 38: 30–5
Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001; 285: 437–43
Mathieu S, Boutron I, Moher D, et al. Comparison of registered published primary outcomes in randomized controlled trials. JAMA 2009; 302: 977–84
Lau J, Ioannidis JP, Schmid CH. Summing up evidence: one answer is not always enough. Lancet 1998; 351: 123–7
Acknowledgements
We would like to express our appreciation to the Thai Pharmacy Network for Tobacco Control for their financial support. We would also like to thank Assistant Professor Surakit Nathisuwan for his contribution in editing this manuscript. The authors of this study have nothing to disclose concerning possible financial or personal relationships with commercial entities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhippayom, T., Chaiyakunapruk, N. & Jongchansittho, T. Safety of Nortriptyline at Equivalent Therapeutic Doses for Smoking Cessation. Drug-Safety 34, 199–210 (2011). https://doi.org/10.2165/11585950-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11585950-000000000-00000