Abstract
This article discusses seven newly available antiepileptic drugs (AEDs) and agents in phase III development.
Lacosamide, licensed as an adjunctive treatment for partial-onset seizures, primarily acts by enhancing sodium channel slow inactivation. At daily doses of 200–600 mg, the drug significantly reduced partial-onset seizures in adults with refractory epilepsy. The most common adverse effects are CNS related.
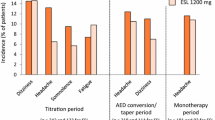
Rufinamide, available as adjunctive treatment for seizures associated with Lennox-Gastaut syndrome, has an unclear mechanism of action, although it does block voltage-dependent sodium channels. Coadministration of valproic acid significantly increases rufinamide circulating concentrations. The drug has been shown to have efficacy for partial-onset, primary generalized tonic-clonic, tonic-atonic, absence and atypical absence seizures. Adverse effects are mainly somnolence, nausea and vomiting.
Eslicarbazepine acetate, a carbamazepine analogue, was recently licensed as adjunctive treatment for partial-onset seizures. Eslicarbazepine acetate acts at voltage-gated sodium channels, although the precise mechanism of action is unclear. The drug had efficacy for partial-onset seizures in three randomized, double-blind, placebo-controlled studies, using 400, 800 or 1200mg/day. Adverse effects include dizziness and somnolence.
Retigabine (ezogabine) exerts its anticonvulsant effect through the opening of neuronal voltage-gated potassium channels. Following significant seizure reduction rates at dosages of 600, 900 and 1200mg/day, license applications have been submitted for its use as adjunctive treatment for patients with partial-onset seizures. Dose-related adverse effects include somnolence, confusion and dizziness.
Brivaracetam is the n-propyl analogue of levetiracetam. Mixed results have been obtained in phase III studies in patients with partial-onset seizures, and further trials in children, patients with photosensitive epilepsy and patients with partial-onset seizures are ongoing. Dizziness, headache and somnolence are the most common adverse effects reported.
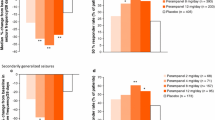
Perampanel was designed as an AMPA-type glutamate receptor antagonist. Following encouraging results from phase II studies in patients with refractory partial-onset seizures, recruitment for phase III trials is almost complete.
Ganaxolone is a neurosteroid with potent antiepileptic activity that modulates GABAA receptors in the CNS. Ganaxolone has shown promise in a variety of seizure types. Dizziness and somnolence have been reported in some patients.
The availability of new AEDs has widened the choices for clinicians treating patients with epilepsy. However, given the minimal improvement in prognosis and disappointing efficacy outcomes in double-blind, placebo-controlled, dose-ranging regulatory trials, it seems unlikely that these novel agents will have a major impact on outcomes for people with epilepsy.

Similar content being viewed by others
References
Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 1996; 71: 576–86
Kwan P, Brodie MJ. Refractory epilepsy: mechanisms and solutions. Expert Rev Neurotherapeutics 2006; 6: 397–406
Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 51: 1069–77
Beyreuther BK, Freitag J, Heers C, et al. Lacosamide: a review of preclinical properties. CNS Drug Rev 2007; 13: 21–42
Errington AC, Stöhr T, Heers C, et al. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol 2008; 73: 157–69
Binder DK. The role of BDNF in epilepsy and other diseases of the mature nervous system. Adv Exp Med Biol 2004; 548: 34–56
Tongiori E, Domenici L, Simonato M. What is the biological significance of BDNF mRNA targeting in the dendrites? Clues from epilepsy and cortical development. Mol Neurobiol 2006; 33: 17–32
Charrier E, Reibel S, Rogemond V, et al. Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Mol Neurobiol 2003; 28: 51–63
Brandt C, Heile A, Potschka H, et al. Effects of the novel antiepileptic drug lacosamide on the development of amygdala kindling in rats. Epilepsia 2006; 47: 1803–9
Duncan GE, Kohn H. The novel antiepileptic drug lacosamide blocks behavioural and brain metabolic manifestations of seizure activity in the 6 Hz psychomotor seizure model. Epilepsy Res 2005; 67: 81–7
Biton V, Rosenfeld WE, Whitesides J, et al. Intravenous lacosamide as replacement for oral lacosamide in patients with partial-onset seizures. Epilepsia 2008; 49: 123–4
Kropeit D, Schiltmeyer B, Cawello W, et al. Bioequivalence od short-time infusions compared to oral administration of SPM927 [abstract]. Epilepsia 2004; 45 Suppl. 7: 123
Bialer M, Johannessen SI, Kepferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the Seventh Eilat Conference (EILAT VII). Epilepsy Res 2004; 61: 1–48
Horstmann R, Bonn R, Cawello W, et al. Basic clinical pharmacological investigations of the new antiepileptic drug SPM 927 [abstract]. Epilepsia 2002; 43 Suppl. 7: 188
Bialer M, Johannessen SJ, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the Eight Eilat Conference (EILAT VIII). Epilepsy Res 2007; 73: 1–52
Cross SA, Curran MP. Lacosamide in partial-onset seizures. Drugs 2009; 69: 449–59
Vimpat® (lacosamide): US prescribing information. Smyrna (GA): UCB, Inc., 2008 [online]. Available from URL: http://www.vimpat.com/hcp/pdfs/VIMPAT%20PI.pdf [Accessed 2010 Oct 13]
Ben-Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 2007; 48: 1308–17
Halasz P, Kalviainen R, Mazurkiewicz-Beldzinska M, et al. Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia 2009; 50: 443–53
Chung SS, Sperling M, Biton V, et al., on behalf of the SP754 Study Group. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia 2010; 51: 958–67
Shaibani A, Fares S, Salah S, et al. Lacosamide in painful diabetic neuropathy: an 18-week double-blind placebo-controlled trial. J Pain 2009; 10: 818–28
Wymer JP, Simpson J, Sen D, et al. Efficacy and safety of lacosamide in diabetic neuropathic pain: an 18-week double-blind, placebo-controlled trial of fixed-dose regimens. Clin J Pain 2009; 25: 376–85
Kennebäck G, Bergfeldt L, Vallin H, et al. Electrophysiologic effects and clinical hazards of carbamazepine treatment for neurologic disorders in patients with abnormalities of the cardiac conduction system. Am Heart J 1991; 121: 1421–9
Matsuo F, Bergen D, Faught E, et al. Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures: US Lamotrigine Protocol 0.5 Clinical Trial Group. Neurology 1993; 43: 2284–91
Kennebäck G, Bergfeldt L, Tomson T. Electrophysiological evaluation of the sodium-channel blocker carbamazepine in healthy human subjects. Cardiovasc Drugs Ther 1995; 9: 709–14
Laville MA, de la Gastine B, Husson B, et al. Should we care about pregabalin for elderly patients with a history of cardiac dysrhythmia? Rev Med Interne 2008; 29: 152–4
DeGiorgio CM. Atrial flutter/atrial fibrillation associated with lacosamide for partial seizures. Epilepsy Behav 2010; 18: 322–4
Fountain NB, Krauss G, Isojarvi J, et al. A multicenter, open-label trial to assess the safety and tolerability of a single intravenous loading dose of lacosamide followed by oral maintenance as adjunctive therapy in patients with partial-onset seizures [abstract]. Epilepsia 2010; 51 Suppl. 4: 123
White HS, Franklin MR, Kupferberg HJ, et al. The anti-convulsant profile of rufinamide (CGP 33101) in rodent seizure models. Epilepsia 2008; 49: 1213–20
Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the Fifth Eilat Conference (EILAT V). Epilepsy Res 2001; 43: 11–58
Arroyo S. Rufinamide. Neurotherapeutics 2007; 4: 155–62
Perucca E, Cloyd J, Critchley D, et al. Rufinamide: clinical pharmacokinetics and concentration-response relationships in patients with epilepsy. Epilepsia 2008; 49: 1123–41
Eisai. Banzel™ (rufinamide) tablets: summary of product characteristics [online]. Available from URL: https://www.eisaimedicalinformation.com/emi/docs/Banzel_PI.pdf [Accessed 2010 Sep 23]
Fuseau E, Petricoul O, Critchley D, et al. Population pharmacokinetic drug-drug interaction analyses of rufinamide in patients with epilepsy [abstract]. Epilepsia 2005; 46 Suppl. 8: 210
Critchley D, Fuseau E, Perdomo C, et al. Pharmacokinetic and pharmacodynamic parameters of adjunctive rufinamide in patients with Lennox-Gastaut syndrome [abstract]. Epilepsia 2005; 46 Suppl. 8: 209
Racine A, Rouan MC, Chang SW, et al. Population pharmacokinetics and drug-drug interactions of rufinamide in a multicenter, double-blind, randomized, placebo-controlled, 5-arm parallel trial in patients with partial seizures on up to three concomitant antiepileptic drugs. Epilepsia 2000; 41 Suppl. : 149–50
Palhagan S, Canger R, Henriksen O, et al. Rufinamide: a double-blind, placebo-controlled proof of principle trial in patients with epilepsy. Epilepsy Res 2001; 43: 115–24
Glauser T, Kluger G, Sachdeo R, et al. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Ann Neurol 2008; 70: 1950–8
Brodie MJ, Rosenfeld WE, Vazquez B, et al. Rufinamide for the adjunctive treatment of partial seizures in adults and adolescents: a randomized placebo-controlled trial. Epilepsia 2009; 50: 1899–909
Elger CE, Hermann S, Mann A, et al. A 24-week multicenter, randomized, double-blind, parallel-group, dose-ranging study of rufinamide in adults and adolescents with inadequately controlled partial seizures. Epilepsy Res 2010; 88: 255–63
Kluger G, Kurlemann G, Haberlandt E, et al. Effectiveness and tolerability of rufinamide in children and adults with refractory epilepsy: first European experience. Epilepsy Behav 2009; 14: 491–5
Hancock EC, Cross HJ. Treatment of Lennox-Gastaut syndrome. Cochrane Database Syst Rev 2009; (3): CD003277
Aldenkamp AP, Alpherts WC. The effect of the new antiepileptic drug rufinamide on cognitive function. Epilepsia 2006; 47: 1153–9
Benes J, Parada A, Figueiredo AA, et al. Anticonvulsant and sodium channel-blocking properties of novel 10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide derivatives. J Med Chem 1999; 42: 2582–7
Bonifacio MJ, Sheriden RD, Parada A, et al. Interaction of the novel anticonvulsant, BIA 2-093 with voltage-gated sodium channels: comparison with carbamazepine. Epilepsia 2001; 42: 600–8
Parada A, Soares-Da-Silva P. The novel anticonvulsant BIA 2-093 inhibits transmitter release during opening of voltage-gated sodium channels: a comparison with carbamazepine and oxcarbazepine. Neurochem Int 2002; 40: 435–40
Hainzl D, Parada A, Soares-da-Silva P. Metabolism of two new antiepileptic drugs and their principal metabolites S(+) and R(−)-10,11-,dihydro-10-hydroxy carbamazepine. Epilepsy Res 2001; 44: 197–206
Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res 2006; 69: 273–94
Almeida L, Soares-da-Silva P. Eslicarbazepine acetate (BIA 2-093). Neurother 2007; 4: 88–96
Almeida L, Potgieter JH, Maia J, et al. Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment. Eur J Clin Pharmacol 2008; 64: 267–73
McCormack PL, Robinson DM. Eslicarbazepine acetate. CNS Drugs 2009; 23: 71–9
Maia J, Almeida L, Falcao A, et al. Effect of renal impairment on the pharmacokinetics of eslicarbazepine acetate. Int J Clin Pharmacol Ther 2008; 46: 119–30
European Medicines Agency. Zebinix: summary of product characteristics [online]. Available from URL: http://www.medicines.org.uk/EMC/medicine/22376/SPC/Zebinix+800mg+tablets/ [Accessed 2010 Oct 13]
Perucca E, Falcao A, Maia J, et al. Assessment of the impact of eslicarbazepine acetate on carbamazepine pharmacokinetics at steady-state: a pooled analysis of three placebo-controlled phase III studies [abstract]. Epilepsia 2010; 51 Suppl. 4: 122
Nunes T, Sicard E, Almeida L, et al. Pharmacokinetic interaction study between eslicarbazepine acetate and topiramate in healthy subjects. Curr Med Res Opin 2010; 26: 1355–62
Hufnagel A, Ben-Menachem E, Gabbai AA, et al. Efficacy and safety of eslicarbazepine acetate as add-on treatment in adults with refractory partial-onset seizures: BIA-2093-302 study [abstract]. Epilepsia 2008; 50 Suppl. 4: 104
Elger C, Halácz P, Maia J, et al., on behalf of the BIA-2093-301 Investigators Study Group. Efficacy and safety of eslicarbazpine acetate in adults with refractory partial-onset seizures: a randomized double-blind, placebo-controlled, parallel group phase III study. Epilepsia 2009; 50: 454–63
Gil-Nagel A, Lopes-Lima J, Almeida L, et al., on behalf of the BIA-2093-303 Investigators Study Group. Efficacy and safety of 600 and 1200mg eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures. Acta Neurol Scand 2009; 120: 281–7
Elger C, Bialer M, Cramer JA, et al. Eslicarbazepine acetate: a double-blind add-on, placebo-controlled exploratory trial in adult patients with partial-onset seizures. Epilepsia 2007; 48: 497–504
Halász P, Cramer JA, Hodoba D, et al., on behalf of the BIA-2093-301 Study Group. Long-term efficacy and safety of eslicarbazepine acetate: results of a 1-year open-label extension study in partial-onset seizures in adults with epilepsy. Epilepsia 2010 Oct; 51(10): 1963–9
Cramer J, Hodoba D, Ben-Menachem E, et al. Depressive symptoms improve with 1-year eslicarbazepine acetate treatment: a pooled analysis of 3 open-label extensions of phase III studies in patients with partial-onset seizures [abstract]. Epilepsia 2010; 51 Suppl. 4: 117
Bialer M, Johannessen S, Levy R, et al. Progress report on new antiepileptic drugs: a summary of the Ninth Eilat Conference (EILAT IX). Epilepsy Res 2009; 83: 1–43
Chung SS. Third-generation antiepileptic drugs for partial-onset seizures: lacosamide, retigabine, and eslicarbazepine acetate. Eur J Neurol 2009; 1: 1–11
Rundfeldt C, Netzer R. The novel anticonvulsant retigabine activates M-currents in Chinese hamster ovary-cells tranfected with human KCNQ2/3 subunits. Neurosci Lett 2000; 282: 73–6
Rogawski MA. KCNQ2/KCNQ3 K+ channels and the molecular pathogenesis of epilepsy: implications for therapy. Trends Neurosci 2000; 23: 393–8
Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 2005; 6: 850–62
Maljevic S, Wuttke TV, Lerche H. Nervous system KV7 disorders: breakdown of a subthreshold brake. J Physiol 2008; 586: 1791–801
Wuttke TV, Seebohm G, Bail S, et al. The new anticonvulsant retigabine favors voltage-dependent opening of the Kv7.2 (KCNQ2) channel by binding to is activation gate. Mol Pharmacol 2005; 67: 1009–17
Schenzer A, Friedrich T, Pusch M, et al. Molecular determinants of KCNQ (Kv7) K+ channel sensitivity to the anticonvulsant retigabine. J Neurosci 2005; 25: 5051–60
Lange W, Geissendörfer J, Schenzer A, et al. Refinement of the binding site and mode of action of the anticonvulsant retigabine on KCNQ K+ channels. Mol Pharmacol 2009; 75: 272–80
Bievert C, Schroeder BC, Kubisch C, et al. A potassium channel mutation in neonatal human epilepsy. Science 1998; 279: 403–6
Singh NA, Charlier C, Stauffer D, et al. A novel potassium channel gene, KCN2, is mutated in an inherited epilepsy of newborns. Nat Genet 1998; 18: 25–9
Charlier C, Singh NA, Ryan SG, et al. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998; 18: 53–5
Jentsch TJ, Schroeder BC, Kubisch C, et al. Pathophysiology of KCNQ channels: neonatal epilepsy and progressive deafness. Epilepsia 2000; 41: 1068–9
Neubauer BA, Waldegger S, Heinzinger J, et al. KCNQ2 and KCNQ3 mutations contribute to different idiopathic epilepsy syndromes. Neurology 2008; 71: 177–83
Meldrum BS, Rogawski MA. Molecular targets for anti-epileptic drug development. Neurotherapeutics 2007; 4: 18–61
Benarroch EE. Potassium channels: brief overview and implications in epilepsy. Neurology 2009; 72: 664–9
Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol 2003; 460: 109–16
Korsgaard MP, Hartz BP, Brown WD, et al. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther 2005; 314: 282–92
Dencker D, Dias R, Pedersen ML, et al. Effect of the new antiepileptic drug retigabine in a rodent model of mania. Epilepsy Behav 2008; 12: 49–53
Tatulian L, Delmas P, Abogadie FC, et al. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci 2001; 21: 5535–45
Dailey JW, Cheong JH, Ko KH, et al. Anticonvulsant properties of D-20443 in genetically epilepsy-prone rats: prediction of clinical response. Neurosci Lett 1995; 195: 77–80
Tober C, Rostock A, Bartsch R. D-23129: a potent anticonvulsant in the amygdala kindling model of complex partial seizures. Eur J Pharmacol 1996; 303: 163–9
Rostock A, Tober C, Rundfeldt C, et al. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res 1996; 23: 211–23
Sotty F, Damgaard T, Montezinho L, et al. Antipsychotic-like effect of retigabine [N-(2-amino-4-(fluorobenzylamino)-phenyl) carbamic acid ester]: a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. J Pharmacol Exp Ther 2009; 328: 951–62
Roza C, Lopez-Garcia JA. Retigabine, the specific KCNQ channel opener, blocks ectopic discharges in axomitized sensory fibres. Pain 2008; 138: 537–45
Hermann R, Ferron GM, Erb K, et al. Effects of age and sex on the disposition of retigabine. Clin Pharmacol Ther 2003; 73: 61–70
Ferron GM, Paul J, Fruncillo R, et al. Multiple-dose, linear dose-proportional pharmacokinetics of retigabine in healthy volunteers. J Clin Pharmacol 2002; 42: 175–82
Ferron GM, Patat A, Parks V, et al. Lack of pharmacokinetic interaction between retigabine and phenobarbitone at steady-state in healthy subjects. Br J Clin Pharmacol 2003; 56: 39–45
McNeilly PJ, Torchin CD, Andreson LW, et al. In vitro glucuronidation of D-23129, a new anticonvulsant, by human liver microsomes and liver slices. Xenobiotica 1997; 5: 431–41
Hempel R, Schupke H, Mcneilly PJ, et al. Metabolism of retigabine (D-23129), a novel anticonvulsant drug. Drug Metab Dispos 1999; 27: 613–22
Brodie MJ, Lerche H, Gil-Nagel A, et al., on behalf of the RESTORE 2 Study Group. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. Epub 2010 Oct 13
Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: a summary of the Fourth Eilat Conference (EILAT IV). Epilepsy Res 1999; 34: 1–41
Hovinga CA. SPM-927 (Schwarz Pharma). Drugs 2003; 6: 479–85
Tompson DJ, VanLandingham KE. The effects of retigabine on the pharmacokinetics of concomitantly administered antiepileptic drugs [abstract]. Epilepsia 2010; 51 Suppl. 4: 123
Ferron GM, Sachdeo R, Partiot A, et al. Pharmacokinetic interaction between valproic acid, topiramate, phenytoin or carbamazepine and retigabine in epileptic patients [abstract]. Clin Pharmacol Ther 2001; 63: 18
Hermann R, Knebel NG, Niebch G, et al. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol 2003; 58: 795–802
Paul J, Ferron GM, Richards L, et al. Retigabine does not alter the pharmacokinetics of a low-dose oral contraceptive in women. Neurology 2001; 56 Suppl. 3: A335–6
Porter RJ, Partiot A, Sachdeo R, et al. Randomized, multicenter, dose-ranging trial of retigabine for partial-onset seizures. Neurology 2007; 68: 1197–204
French J, Mansbach H, RESTORE 1 Investigators. 1200mg/day retigabine as adjunctive therapy in adults with refractory partial-onset seizures. Epilepsia 2008; 49: 112–3
Kushida CA, Becker PM, Ellenbogen AL, et al. Randomized, double-blind, placebo-controlled study of XP13512/ GSK1838262 in patients with RLS. Neurology 2009; 72: 439–46
Kwan P, Brodie MJ. Emerging drugs for epilepsy. Expert Opin Emerg Drugs 2007; 12: 407–22
Kenda BM, Matagne A, Talaga PE, et al. Discovery of 4 substituted pyrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem 2004; 47: 530–49
Niespoziany I, Leclere N, Matagne A, et al. Brivaracetam modulates Na+ currents expressed in a neuroblastoma cell line: comparison with carbamazepine. Epilepsia 2010; 51 Suppl. 4: 149–50
Matagne A, Margineau DG, Kenda B, et al. Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br J Pharmacol 2008; 154: 1662–71
Von Rosensteil P. Brivaracetam (ucb 34714). Neurotherapeutics 2007; 4: 84–7
Margineau DG, Klitgaard H. Brivaracetam inhibits spreading depression in rat neocortical slices in vitro. Seizure 2009; 18:453–6
Rolan P, Sargentini-Maier ML, Pigeolet E, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after multiple increasing oral doses in healthy men. Br J Clin Pharmacol 2008; 66: 71–5
Sargentini-Maier ML, Rolan P, Connell J, et al. The pharmacokinetics, CNS pharmacodynamics and adverse event profile of brivaracetam after single increasing oral doses in healthy males. Br J Clin Pharmacol 2007; 63: 680–8
Sargentini-Maier ML, Espié P, Coquette A, et al. Pharmacokinetics and metabolism of [14C]-brivaracetam, a novel SV2A ligand, in healthy subjects. Drug Metab Dispos 2008; 36: 36–45
French JA, Costantini C, Brodsky A, et al., on behalf of the N01193 Study Group. Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology 2010; 75: 519–25
Van Paesschen W, von Rosentiel P. Efficacy and tolerability of brivaracetam as adjunctive treatment for adults with refractory partial-onset epilepsy: platform session 064. 27th International Epilepsy Congress; 2007 Jul 8–12; Singapore
Werkahn KJ, Biton V, Johnson ME, et al., on behalf of the N01252 and N01253 Brivaracetam Study Groups. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from two randomized, double-blind, placebo-controlled trials [abstract]. Epilepsia 2010; 51 Suppl. 4: 150
Kasteleijn-Nolst Trenite DG, Genton P, Parain D, et al. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology 2007; 69: 1027–34
Luszczki JL. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharm Rep 2009; 61: 197–216
Kwan P, Johnson ME, Merschemke M, et al., on behalf of the N01254 Brivaracetam Study Group. Safety and tolerability of adjunctive brivaracetam in adults with uncontrolled epilepsy: randomized, double-blind, placebo-controlled trial. [abstract]. Epilepsia 2010; 51 Suppl. 4: 152
Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov 2010; 9: 68–82
US National Institutes of Health. Perampanel [online]. Available from URL: http://www.clinicaltrials.gov/ct2/results?term=perampanel [Accessed 2010 Sep 23]
Rogawski MA, Donevan SD. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv Neurol 1999; 79: 947–63
Rogawski MA, Kurzman PS, Yamaguchi SI, et al. Role of AMPA and GluR5 kainate receptors in the development and expression of amygdala kindling in the mouse. Neuropharmacology 2001; 40: 28–35
Gottwald MF, Aminoff MJ. New frontiers in the pharmacological management of Parkinson’s. Drugs Today 2008; 44: 531–45
Eisai Co Ltd. Status of the E2007 (perampanel) Development Program: termination of Parkinson’s disease clinical development and focus on neuropathic pain and epilepsy indications [media release]. 2008 Apr 11 [online]. Available from URL: http://www.eisai.co.jp/enews/enews200819.html [Accessed 2010 Sep 23]
Hashizume Y, Hanada T, Ogassawara A, et al. Anti-convulsant activity of perampanel, a selective AMPA receptor antagonist, in rodent models of epileptic seizure [abstract no. P02.113]. American Academy of Neurology Meeting; 2008 Apr 12–19; Chicago (IL). Available from URL: http://www.abstracts2view.com/aan2008chicago/view.php?nu=AAN08L_P02.113 [Accessed 2010 Sep 23]
Templeton D. Pharmacokinetics of peramapanel, a highly selective AMPA-type glutamate receptor antagonist following once- and multiple-daily dosing [abstract]. Epilepsia 2010; 51 Suppl. 4: 70
Krauss G, Yang J, Biton V, et al. Determination of maximum tolerated dose (MTD), safety, efficacy, and pharmacokinetics (PK) of perampanel, a selective AMPA receptor antagonist, as adjunctive therapy in subjects with refractory partial seizures [abstract]. Epilepsia 2008; 49 Suppl. 7: 1.106 [online]. Available from URL: http://www.aesnet.org/go/publications/aes-abstracts/abstract-search/mode/display/st/krauss/sy/2008/sb/Authors/id/8447 [Accessed 2010 Oct 13]
Eisai Co. Ltd. Status of the E2007 (perampanel) Development Program: termination of Parkinson’s disease clinical development and focus on neuropathic pain and epilepsy indications [media release]. 2008 Apr 11 [online]. Available from URL: http://www.eisai.co.jp/enews/enews200819.html [Accessed 2010 Oct 13]
US National Institute of Health. Evaluating efficacy and safety of E2007 (perampanel) given as adjunctive therapy in subjects with refractory partial seizures [online]. Available from URL: http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00700310 [Accessed 2010 Jul]
Ganaxolone (CCD 1042): investigational drug brochure. Irvine (CA): CoCensys Inc., 1996
Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (Metrazol). J Lab Clin Med 1942; 27: 1051–3
Figdor SK, Kodet MJ, Bloom BM, et al. Central activity and structure in a series of water-soluble steroids. J Pharmacol Exp Ther 1957; 119: 299–309
Craig CR. Antiepileptic activity of steroids: separability of antiepileptic from hormonal effects. J Pharmacol Exp Ther 1966; 153: 337–43
Gee KW, McCauley LD, Nan LC. A putative receptor for neurosteroids on the GABAA receptor complex: the pharmacological properties and therapeutic potential of epalons. Crit Rev Neurobiol 1995; 9: 207–27
Carter RB, Wood PL, Wieland S, et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective high-affinity steroid modulator of the GABAA receptor. J Pharmacol Exp Ther 1997; 280: 1284–95
Gasior M, Carter RB, Goldberg SR, et al. Anticonvulsant and behavioral effects of neuroactive steroids alone and in conjunction with diazepam. J Pharmacol Exp Ther 1997; 282: 543–53
Beekman M, Ungard JT, Gasior M, et al. Reversal of behavioural effects of pentylenetetrazol by the neuroactive steroid ganaxolone. J Pharmacol Exp Ther 1998; 284: 868–77
Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res 2009; 85: 1–30
Robichaud M, Debonnel G. Allopregnanolone and ganaxolone increase the firing activity of dorsal raphe nucleus serotonergic neurons in female rats. Int J Neuropsychopharmacol 2006; 9: 191–200
Monaghan EP, Navalta LA, Shum L, et al. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia 1997; 38: 1026–31
Nohria V, Giller E. Ganaxolone. Neurotherapeutics 2007; 4: 102–5
Pieribone VA, Tsai J, Soufflet C, et al. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia 2007; 48: 1870–4
Kerrigan JF, Shields WD, Nelson TY, et al. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res 2000; 42: 133–9
Laxer K, Blum D, Abou-Khalil BW, et al. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Epilepsia 2000; 41: 1187–94
McAuley JW, Moore JL, Reeves AL, et al. A pilot study of the neurosteroid ganaxolone in catamenial epilepsy: clinical experience in two patients [abstract]. Epilepsia 2001; 42 Suppl. 7: 85
Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. In press
Callaghan BC, Anand K, Hesdorffer D, et al. Likelihood of seizure remission in adult population with refractory epilepsy. Ann Neurol 2007; 62: 382–9
Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol 2007; 62: 375–81
Schiller Y, Najjar Y. Quantifying response to antiepileptic drugs: effect of past treatment testing. Neurology 2008; 70: 54–65
Brodie MJ, Bamagous G, Kwan P. Improved outcomes in newly diagnosed epilepsy. Epilepsia 2009; 50 Suppl. 11: 411–2
Beyenburg S, Stavern K, Schmidt D. Placebo-corrected efficacy of modern antiepileptic drugs for refractory epilepsy: systematic review and meta-analysis. Epilepsia 2010; 51: 7–26
Gazzola DM, Balcer LJ, French JA. Seizure-free outcomes in randomized add-on trials of new antiepileptic drugs. Epilepsia 2007; 48: 1303–7
Acknowledgements
Prof. Brodie serves on the scientific advisory boards of Pfizer, UCB Pharma, Eisai, GlaxoSmithKline, Valeant Pharmaceuticals, Sierra Neuropharmaceuticals and Medtronic. He has accepted honoraria for speaking on behalf of Pfizer, UCB, Eisia, GlaxoSmithKline and Valeant Pharmaceuticals. He has received research grants from Pfizer, UCB Pharma and Eisai within the past 3 years. Dr Stephen has accepted honoraria for speaking on behalf of Eisai, GlaxoSmithKline and Sanofi-Aventis. No sources of funding were used to prepare this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stephen, L.J., Brodie, M.J. Pharmacotherapy of Epilepsy. CNS Drugs 25, 89–107 (2011). https://doi.org/10.2165/11584860-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11584860-000000000-00000