Abstract
Background: In clinical trials and non-interventional studies encompassing >50 000 women, the monophasic, low-dose combined oral contraceptive (OC) chlormadinone acetate 2 mg/ethinylestradiol 0.03 mg (CMA/EE) has been shown to have various non-contraceptive benefits, as well as contraceptive efficacy and good tolerability. However, there is a paucity of data on use of this OC in young women.
Objective: To investigate the relevance of, and changes in, cycle disorders, dysmenorrhoea and skin problems in addition to the efficacy and tolerability of CMA/EE in young women.
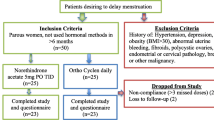
Methods: In this prospective, observational, non-interventional, multicentre study (TeeNIS [Teenager in Non-Interventional Study 2mg CMA/0.03mgEE]), young women (≤20 years of age) were administered CMA/EE (Belara®) once daily for 21 days (one blister strip), followed by either a 7-day pill-free interval (conventional cycle regimen; 89.3%) or a pill-free interval after two blister strips or more (extended cycle regimen; 3.7%), over a 6-month treatment period. Data on the mode of administration were missing for 7.1% of patients. The study included a safety population of 7462 patients (the efficacy population consisted of 6885 patients) from 886 gynaecological centres throughout Germany.
Results: Compared with baseline, CMA/EE intake resulted in significant reductions in the numbers of patients with cycle disorders, i.e. spotting (−46%), breakthrough bleeding (−64%), heavy bleeding (−95%) and absence of any bleeding (secondary amenorrhoea; −76%) [all p≤ 0.001], and with dysmenorrhoea (−56%) [p≤ 0.001]. Similarly, there was a significant decrease in the number of patients who used analgesics (−75%), had dysmenorrhoea-associated symptoms (back pain [−69%], headache [−70%], nausea/vomiting [−85%], diarrhoea [−80%], mood swings [−75%] or absence from school/job due to dysmenorrhoea [−92%]), or were restricted in their leisure/sporting activities because of dysmenorrhoea (−83%) [all p ≤ 0.001]. Another major benefit of CMA/EE was a significant reduction in the number of patients with skin problems (acne and acne-prone skin) [−55%; p≤ 0.001]. In parallel, the number of patients who needed dermatological treatment (−67%; p≤ 0.001) and concealer cosmetics (−55%; p≤ 0.001) was significantly reduced, and significantly fewer patients felt that their self-esteem was restricted due to skin problems (−67%; p≤ 0.001). There were no relevant weight changes during the observation period; mean bodyweight remained virtually constant (mean weight change <1 kg). At final assessment, physicians’ expectations were either ‘completely fulfilled’ or ‘exceeded’ with regard to cycle stability, regular bleeding, dysmenorrhoea, effects on weight, and skin problems in 78–95% of patients. CMA/EE provided high contraceptive efficacy with an unadjusted Pearl index of 0.25, calculated from 41 601 cycles of exposure; seven out of eight pregnancies were attributable to user failure, thus resulting in an adjusted Pearl index of 0.03. The tolerability of CMA/EE was excellent, with no unexpected adverse effects.
Conclusions: This observational, non-interventional study in young women showed that CMA/EE had a significantly beneficial effect on cycle disorders, dysmenorrhoea and skin disorders, and confirmed the good efficacy and tolerability of this combined OC.







Similar content being viewed by others
References
Mosher WD, Martinez GM, Chandra A, et al. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data 2004; (350): 1-36
Hickey M, Balen A. Menstrual disorders in adolescence: investigation and management. Hum Reprod Update 2003; 9: 493–504
Slap GB. Menstrual disorders in adolescence. Best Pract Res Clin Obstet Gynaecol 2003; 17: 75–92
Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhoea. Pediatrics 1981; 68: 661–4
Ziv A, Boulet JR, Slap GB. Utilization of physician offices by adolescents in the United States. Pediatrics 1999; 104: 35–42
Neinstein LS. Menstrual problems in adolescents. Med Clin North Am 1990; 74: 1181–203
Bergfeld WF. The evaluation and management of acne: economic considerations. J Am Acad Dermatol 1995; 32: S52–6
Zahradnik HP, Goldberg J, Andreas JO. Efficacy and safety of the new antiandrogenic oral contraceptive Belara®. Contraception 1998; 57: 103–9
Worret I, Arp W, Zahradnik HP, et al. Acne resolution rates: results of a single-blind, randomised, controlled, parallel phase III trial with EE/CMA (Belara®) and EE/LNG (Microgynon®). Dermatology 2001; 203: 38–44
Schramm G, Steffens D. Contraceptive efficacy and tolerability of chlormadinone acetate 2 mg/ethinylestradiol 0.03 mg (Belara®). Clin Drug Investig 2002; 22: 221–31
Schramm G, Steffens D. A 12-month evaluation of the CMA-containing oral contraceptive Belara®: efficacy, tolerability and anti-androgenic properties. Contraception 2003; 67: 305–12
Zahradnik HP. Belara® — a reliable oral contraceptive with additional benefits for health and efficacy in dysmenorrhoea. Eur JContracept Reprod Health Care 2005; 10Suppl. 1:12–8
Kerscher M, Reuther T, Bayrhammer J, et al. Effect of an oral contraceptive containing chlormadinone and ethinyl-estradiol on acne-prone skin of women of different age groups. Clin Drug Investig 2008; 28(11): 703–11
Schramm G, Heckes B. Switching hormonal contraceptives to a chlormadinone acetate-containing oral contraceptive: the Contraceptive Switch Study. Contraception 2007; 76: 84–90
Feige A, Rempen A, Würfel W, et al. Frauenheilkunde: Fortpflanzungsmedizin Geburtsmedizin Onkologie Psychosomatik (Gebundene Ausgabe). In: Geisthövel F, editor. Munich: Elsevier GmbH, 2005
Runnebaum B, Rabe T. Gynäkologische Endokrinologie. Berlin: Springer-Verlag, 1994: 413
Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Nelson AL, et al., editors. Contraceptive technology. 19th rev ed. New York: Ardent Media, 2007
Spona J, Binder N, Höschen K, et al. Contraceptive efficacy and safety of a low-dose oral contraceptive (0.03 mg ethinyl oestradiol and 2 mg chlormadinone acetate) Belara, over three medication cycles. Eur J Contracept Reprod Health Care 2008; 13: 39–48
Vandenbroucke JP, van der Meer FJ, Helmerhorst FM, et al. Factor V Leiden: should we screen oral contraceptive users and pregnant women? BMJ 1996; 313: 1127–30
Cardiovascular disease and steroid hormone contraception: report of a WHO scientific group. WHO Tech Rep Ser 1998; 877: 1–89
Schramm G, Heckes B. The COSS-study: switching hormonal contraceptives to a combined oral contraceptive containing chlormadinone acetate (2 mg) and ethinyl estradiol (0.03 mg) [poster no. P029]. 9th Congress of the European Society of Contraception, 2006 Mar 3–6; Istanbul
Feldman W, Hodgson C, Corber S, et al. Health concerns and health-related behaviours of adolescents. CMAJ 1986; 134:489–93
Hamani Y, Sciaki-Tamir Y, Deri-Hasid R, et al. Misconceptions about oral contraception pills among adolescents and physicians. Hum Reprod 2007; 22: 3078–83
Gallo MF, Nanda K, Grimes DA, et al. 20 mg versus >20 µg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev 2008; 4: CD003989
Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception. In press
Proctor M, Farquhar C. Dysmenorrhoea. Clin Evid 2002; 7: 1639–53
French L. Dysmenorrhea. Am Fam Physician 2005; 71(2): 285–91
Bock K, Heskamp ML, Schramm G. Convincing effect of CMA on dysmenorrhoea and other cycle-related disorders [in German]. Gyne 2008; 8: 219–25
Tan HH. Topical antibacterial treatments for acne vulgaris: comparative review and guide to selection. Am J Clin Dermatol 2004; 5: 79–84
Kerscher M. Hormonal contraception-prospects and limitations in dermatocosmetic indications [in German]. Gynäkol Endokrinol 2009; 7: 17–24
Druckmann R. Profile of the progesterone derivative chlormadinone acetate: pharmacodynamic properties and therapeutic applications. Contraception 2009;79(4): 272–81
Acknowledgements
This study was conducted and sponsored by Grünenthal GmbH, Germany. The authors would like to thank all of the 886 participating investigators. GAKS and M-LSH are employees of Grünenthal GmbH, Germany. SA has no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anthuber, S., Schramm, G.A. & Heskamp, ML.S. Six-Month Evaluation of the Benefits of the Low-Dose Combined Oral Contraceptive Chlormadinone Acetate 2 mg/Ethinylestradiol 0.03 mg in Young Women. Clin. Drug Investig. 30, 211–220 (2010). https://doi.org/10.2165/11532910-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11532910-000000000-00000