Abstract
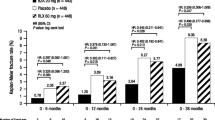
Bazedoxifene (Conbriza®, Viviant®) is the first third-generation selective estrogen receptor modulator (SERM) and it is approved for the treatment of postmenopausal osteoporosis in the EU and Japan. Bazedoxifene contains an indole-based core binding domain that binds with high affinity to estrogen receptors and exhibits favourable effects on bone and lipid profiles, with no clinically relevant endometrial or breast stimulation. Oral bazedoxifene once daily reduced the incidence of new vertebral fractures in patients with postmenopausal osteoporosis in a large, well designed trial of 3 years’ duration; both bazedoxifene and raloxifene were significantly more effective than placebo. Neither bazedoxifene nor raloxifene reduced the incidence of nonvertebral fractures in the overall study population; however, bazedoxifene, but not raloxifene, reduced the rate of nonvertebral fractures in high-risk patients. Moreover, data from patients who continued to receive the drug during a 2-year extension phase of this trial indicate that bazedoxifene continues to provide protection against new vertebral fractures for up to 5 years. Bazedoxifene also increases bone mineral density and reduces the levels of bone turnover markers. Bazedoxifene was generally well tolerated and did not detrimentally affect the reproductive tract or breast tissue in clinical trials, thereby demonstrating a favourable risk-benefit profile. A pharmacoeconomic analysis conducted from an EU perspective predicted bazedoxifene to be cost effective in some EU countries.Therefore, bazedoxifene presents another useful option for the treatment of post-menopausal osteoporosis, especially in those at high risk for osteoporotic fracture.








Similar content being viewed by others
References
Management of osteoporosis in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17(1): 25–54
World Health Organisation. Prevention and management of osteoporosis: report of a WHO scientific group. WHO technical report series [online]. Available from URL: http://whqlibdoc.who.int/trs/who_trs_921.pdf [Accessed 2011 Jul 18]
International Osteoporosis Foundation. Facts and statistics about osteoporosis and its impact [online]. Available from URL: http://www.iofbonehealth.org/facts-and-statistics.html#factsheet-category-14 [Accessed 2011 Jul 18]
Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007 Mar; 22(3): 465–75
Kanis JA, on behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary health care level. Technical report [online]. Available from URL: http://www.sheffield.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf [Accessed 2011 Jul 18]
Kanis JA, Burlet N, Cooper C, et al. European guidance for the diagnosis and management of osteoporosis in post-menopausal women. Osteoporos Int 2008 Apr; 19(4): 399–428
Itabashi A, Yoh K, Chines AA, et al. Effects of bazedoxifene on bone mineral density, bone turnover, and safety in postmenopausal Japanese women with osteoporosis. J Bone Miner Res 2011; 26(3): 519–29
Xu L, Tsai KS, Kim GS. Efficacy and safety of bazedoxifene in postmenopausal Asian women. Osteoporos Int 2011; 22(2): 559–65
International Osteoporosis Foundation. Osteoporosis and you [online]. Available from URL: http://www.iofbonehealth.org/publications/osteoporosis-and-you.html [Accessed 2011 Jul 18]
Felsenberg D, Boonen S. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 2005; 27(1): 1–11
Reginster JY. Antifracture efficacy of currently available therapies for postmenopausal osteoporosis. Drugs 2010; 70(18): 65–78
MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 2008 Feb 5; 148(3): 197–213
National Osteoporosis Foundation. Clinicians guide to the prevention and treatment of osteoporosis [online]. Available from URL: http://www.nof.org/sites/default/files/pdfs/NOF_ClinicianGuide2009_v7.pdf [Accessed 2011 Jul 18]
Gennari L, Merlotti D, Valleggi F, et al. Selective estrogen receptor modulators for postmenopausal osteoporosis: current state of development. Drugs Aging 2007; 24(5): 361–79
Clemett D, Spencer CM. Raloxifene: a review of its use in postmenopausal osteoporosis. Drugs 2000 Aug; 60(2): 379–411
European Medicines Agency. Conbriza 20 mg film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000913/WC500033577.pdf [Accessed 2010 Jul 18]
Viviant tablets 20 mg [package insert]. Tokyo: Pfizer Japan Inc. 2010
US National Institutes of Health. ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2011 Jul 18]
Komm BS, Kharode YP, Bodine PV, et al. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology 2005 Sep; 146(9): 3999–4008
Komm BS, PVN, Minck DR. Effects of bazedoxifene acetate on bone loss: a 12-month study in ovariectomised rats [abstract no M404]. J Bone Miner Res 2007 Sep 1; 22 Suppl. 1: 206
Pfizer Japan Inc. Data on file. 2011
European Medicines Agency. Assessment report for conbriza [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000913/WC500033576.pdf [Accessed 2011 Jul 18]
Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008; 23(12): 1923–34
Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 2008; 23(4): 525–35
Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol 2005; 105(6): 1397–404
Taylor HS. Approaching the ideal selective estrogen receptor modulator for prevention and treatment of post-menopausal osteoporosis. Formulary 2010; 45(2): 52–61
Fujiwara S, Nakamura T, Orimo H, et al. Development and application of a Japanese model of the WHO fracture risk assessment tool (FRAX). Osteoporos Int 2008 Apr; 19(4): 429–35
Orimo H, Hayashi Y, Fukunaga M, et al. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 2001; 19(6): 331–7
Pinkerton JV, Archer DF, Utian WH, et al. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause 2009; 16(6): 1102–8
Archer DF, Pinkerton JV, Utian WH, et al. Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 2009; 16(6): 1109–15
de Villiers TJ, Chines AA, Palacios S, et al. Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int 2011; 22: 567–76
Christiansen C, Chesnut CH, Adachi JD, et al. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskeletal Disorders 2010; 11 (130)
Harvey JA, Holm MK, Ranganath R, et al. The effects of bazedoxifene on mammographic breast density in post-menopausal women with osteoporosis. Menopause 2009; 16(6): 1193–6
Chandrasekaran A, McKeand WE, Sullivan P, et al. Metabolic disposition of [14C] bazedoxifene in healthy, post-menopausal women. Drug Metab Dispos 2009 Jun; 37(6): 1219–25
Shen L, Ahmad S, Park S, et al. In vitro metabolism, permeability, and efflux of bazedoxifene in humans. Drug Metab Dispos 2010 Sep; 38(9): 1471–9
European Medicines Agency. Guideline on the evaluation of medicinal products in the treatment of primary osteoporosis: Committee for Medicinal Products For Human Use (CHMP) [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003405.pdf [Accessed 2011 Jul 18]
Chines AA, Zanchetta JR, Genant HK, et al. Sustained efficacy of bazedoxifene in preventing fractures in post-menopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study [abstract no. OC17]. Osteoporosis Int 2010 May 5; 21 Suppl. 1: S7–24
Kanis JA, Johansson H, Oden A, et al. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 2009 Jun; 44(6): 1049–54
World Health Organisation Collaborating Centre for Metabolic Bone Diseases. FRAX® WHO fracture risk assessment tool [online]. Available from URL: http://www.sheffield.ac.uk/FRAX/ [Accessed 2011 Jul 18]
Borgstrom F, Strom O, Kleman M, et al. Cost-effectiveness of bazedoxifene incorporating the FRAX® algorithm in a European perspective. Osteoporos Int 2011; 22(3): 955–65
Strom O, Borgstrom F, Kleman M, et al. FRAX and its applications in health economics: cost-effectiveness and intervention thresholds using bazedoxifene in a Swedish setting as an example. Bone 2010 Aug; 47(2): 430–7
Miller PD, Derman RJ. What is the best balance of benefits and risks among anti-resorptive therapies for postmenopausal osteoporosis? Osteoporosis Int 2010; 21(11): 1793–802
Pazianas M, Cooper C, Ebitino FH. Long-term treatment with bisphosphonates and their safety in postmenopausal osteoporosis. Ther Clin Risk Manag 2010; 6: 325–43
Lewiecki EM. Safety of long-term bisphosphonate therapy for the management of osteoporosis. Drugs 2011; 71(6): 791–814
Moen MD, Keam SJ. Denosumab: a review of its use in the treatment of postmenopausal osteoporosis. Drugs Aging 2011; 28(1): 63–82
Deeks ED, Dhillon S. Strontium ranelate: a review of its use in the treatment of postmenopausal osteoporosis. Drugs 2010 Apr 16; 70(6): 733–59
Watts NB. Postmenopausal osteoporosis: what’s new and what’s next. Sex Reprod Menopause 2010; 8 (2)
Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002 Jul 17; 288(3): 321–33
Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation [published erratum appears in JAMA 1999 Dec 8; 282 (22): 2124]. JAMA 1999 Jun 16; 281(23): 2189–97
Delmas PD, Genant HK, Crans GG, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 2003 Oct; 33(4): 522–32
Rice VM. Strategies and issues for managing menopause-related symptoms in diverse populations: ethnic and racial diversity. Am J Med 2005; 118 Suppl. 12B: 142–7
Shea JL. Chinese women’s symptoms: relation to menopause, age and related attitudes. Climacteric 2006 Feb; 9(1): 30–9
Melby MK, Lock M, Kaufert P. Culture and symptom reporting at menopause. Hum Reprod Update 2005; 11(5): 495–512
Melby MK. Vasomotor symptom prevalence and language of menopause in Japan. Menopause 2005; 12(3): 250–7
Pinkerton JV, Goldstein SR. Endometrial safety: a key hurdle for selective estrogen receptor modulators in development. Menopause 2010; 17(3): 642–53
European Medicines Agency. Evista 60 mg film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000184/WC500031011.pdf [Accessed 2011 Jul 11]
European Medicines Agency. Fablyn 500 microgram film-coated tablets: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000977/WC500020092.pdf [Accessed 2011 Jul 11]
Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators [published erratum appears in JAMA 1999 Dec 8; 282 (22): 2124]. JAMA 1999 Aug 18; 282(7): 637–45
Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11(1): 83–91
Wyeth Pharmaceuticals. Study evaluating bazedoxifene acetate in osteoporosis in postmenopausal women [ClinicalTrials.gov identifier NCT00205777]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov/ct2/show/NCT00205777?term=bazedoxifene&rank=6 [Accessed 2011 Jul 18]
Kanis JA, Oden A, Johnell O, et al. Uncertain future of trials in osteoporosis. Osteoporos Int 2002; 13(6): 443–9
Schmidt C. Third-generation SERMs may face uphill battle. J Natl Cancer Inst 2010 Nov 17; 102(22): 1690–2
Author information
Authors and Affiliations
Corresponding author
Additional information
An Erratum for this chapter can be found at http://dx.doi.org/10.2165/11631020-000000000-00000
Various sections of the manuscript reviewed by: S.L. Silverman, Division of Medicine and Rheumatology, Cedars-Sinai Medical Center, Beverly Hills, Los Angeles, CA, USA; S.L. Bonnick, Clinical Research Center of North Texas, Denton, TX, USA; M.L. Brandi, Department of Internal Medicine, University of Florence, Florence, Italy; J.A. Kanis, WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield Medical School, Sheffield, UK; D.F. Archer, Department of Obstetrics & Gynecology, Eastern Virginia Medical School, Norfolk, VA, USA.
Data Selection
Sources: Medical literature (including published and unpublished data) on ‘bazedoxifene’ was identified by searching databases since 1996 (including MEDLINE and EMBASE and in-house AdisBase), bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘bazedoxifene’ and (‘postmenopausal osteoporosis’ or ‘postmenopause osteoporosis’). Searches were last updated on 15 July 2011.
Selection: Studies in patients with postmenopausal osteoporosis who received bazedoxifene. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Bazedoxifene, postmenopausal osteoporosis, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, pharmacoeconomics.
An erratum to this article is available at http://dx.doi.org/10.2165/11631020-000000000-00000.
Rights and permissions
About this article
Cite this article
Duggan, S.T., McKeage, K. Bazedoxifene. Drugs 71, 2193–2212 (2011). https://doi.org/10.2165/11207420-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11207420-000000000-00000