Abstract
Objective: To investigate the relative bioavailability and bioequivalence, in fasting and fed conditions, of repeated doses of two omeprazole enteric-coated formulations in healthy volunteers.
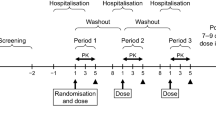
Material and methods: Open label, single-centre study consisting of two consecutive randomised, two-way crossover trials (a fasting trial and a fed trial). Each trial consisted of two 7-day treatment periods in which subjects received one daily dose of the test (Ompranyt®) or reference (Mopral®) formulations. At day 7 and day 14 (fasting trial), products were administered in fasting conditions and blood samples were taken for omeprazole plasma assay over 12 hours. At day 21 and day 28 (fed trial), products were administered after a standard high-calorie and high-fat meal and 12-hour blood samples taken. Omeprazole plasma concentrations were quantified by a validated method using a reverse-phase high performance liquid chromatography with UV detection (HPLC-UV).
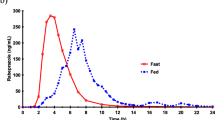
Results: Twenty-four subjects were enrolled and 23 completed the study. Under fasting conditions, the mean ± SD maximum omeprazole plasma concentration (Cmax) was 797 ± 471 μg/L for Ompranyt® and 747 ± 313 μg/L for Mopral® with a point estimate (PE) of 1.01 and a 90% confidence interval (CI) of 0.88, 1.16. The mean ± SD area under the plasma concentration curve from administration to last observed concentration (AUC0–12) was 1932 ± 1611 μg · h/L and 1765 ± 1327 μg · h/L for Ompranyt® and Mopral®, respectively (PE = 1.09; 90% CI 0.95, 1.25). In the presence of food, the Cmax was 331 ± 227 μg/L and 275 ± 162 μg/L (PE = 1.21; 90% CI 0.92, 1.59) and AUC0–12 was 1250 ± 966 μg · h/L and 1087 ± 861 μg · h/L (PE = 1.16; 90% CI 0.92, 1.47) for Ompranyt® and Mopral®, respectively. Bioequivalence of the formulations in the fasting condition was demonstrated both for AUC0–12 and for Cmax because the 90% CI lay within the acceptance range of 0.80–1.25. In contrast with the fasting condition, there were significant reductions in rate (Cmax) and extent (AUC0–12) of systemic exposure when test and reference formulations were administered with food. The food effect was more marked with Mopral® than with Ompranyt®, and the bioequivalence criterion was not fulfilled because the 90% CI fell out of the acceptance range of 0.80, 1.25, for both Cmax and AUC0–12. The two formulations were similarly well tolerated.
Conclusion: Bioequivalence of Ompranyt® (test formulation) and Mopral® (reference) formulations was demonstrated after repeated dosing in the fasting condition. Following a high-calorie and high-fat meal, there was a significant reduction in rate and extent of systemic exposure for both products, with Ompranyt® being less affected than Mopral® by the presence of food.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Howden CW. Clinical pharmacology of omeprazole. Clin Pharmacokinet 1991; 20: 38–49
Sachs G, Shin JM, Besancon M, et al. The continuing development of gastric acid pump inhibitors. Aliment Pharmacol Ther 1993; 7Suppl. 1: 4–12
Guidance for Industry (Draft). Bioavailability and bioequivalence studies for orally administered drug products: general considerations. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2002 Jul
Guidance for Industry (Draft). Food-effect bioavailability and fed bioequivalence studies: study design, data analysis, and labeling. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2001 Oct
Note for guidance on modified release oral and transdermal dosage forms. European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products, CPMP/EWP/280/96 (Draft 15), London, 1999 Jul 28
Andersson T, Andren K, Cederberg C, et al. Bioavailability of omeprazole as enteric coated (EC) granules in conjunction with food on the first and seventh days of treatment. Clin Drug Invest 1990; 2: 184–8
Tolman KG, Sanders SW, Buchi KN, et al. The effects of oral doses of lansoprazole and omeprazole on gastric pH. J Clin Gastroenterol 1997; 24: 65–70
Note for guidance on the investigation of bioavailability and bioequivalence. London: European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products, CPMP/EWP/QWP/1401/98, 2001 Jul 26
Critérios Exigibles a los genéricos de Omeprazol. Comité de Evaluación de Medicamentos de Uso Humano (CODEM), Agencia Española del Medicamento, 2000 Feb 11
Richards JP, Gimeno M, Moreland TA, et al. Estudio de biodisponibilidad relativa de dos formulaciones orales de omeprazol tras la administratción en dosis repetidas a voluntarios sanos. Gastroenterol Hepatol 1999; 22: 171–82
Garg SK, Chugh Y, Tripathi SK, et al. Comparative bioavailability of two enteric-coated capsules of omeprazole in healthy volunteers. Int J Clin Pharmacol Ther Toxicol 1993; 31(2): 96–9
Steinijans VW, Sauter S, Hauschke D, et al. Reference tables for the intrasubject coefficient of variation in bioequivalence studies (short communication). Int J Clin Pharmacol Ther 1995; 33: 427–30
Pillai GK, Salem MS, Najib NM, et al. Bioequivalence study of two capsule formulations of omeprazole. Acta Pharm Hung 1996; 66: 231–5
Farinha A, Bica A, Pais JP, et al. Bioequivalence evaluation of two omeprazole enteric-coated formulations in humans. Eur J Pharm Sci 1999; 7(4): 311–5
Vaz-da-Silva M, Hainzl D, Almeida L, et al. Relative bioavailability of two enteric-coated formulations of omeprazole following repeated doses in healthy volunteers. Clin Drug Invest 2001; 21(3): 203–10
Guidance for Industry. Statistical approaches to establishing bioequivalence. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2001 Jan
USP DI. Drug information to the health care professional, 22nd ed. Omeprazole Systemic. Greenwood Village (CO): Micromedex, 2002: 2210-2213
Schuirmann DJ. A comparison of two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 1987; 15: 657–80
Physicians’ Desk Reference, 57th ed. Prilosec. Montvale: Thomson PDR, 2003: 627–632
Rhoss K, Andren K, Heggelund A, et al. Bioavailability of omeprazole given in conjunction with food. III World Congr Clin Pharmacol Ther, Stockholm July–Aug 1986 [abstract]. Acta PharmacolToxicol 1986; 85Suppl. 5: 207
Stedman CAM, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 2000; 14: 963–78
Acknowledgements
This work was financially supported by BIAL SA. All the authors are employees of BIAL SA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaz-da-Silva, M., Loureiro, A.I., Nunes, T. et al. Bioavailability and Bioequivalence of Two Enteric-Coated Formulations of Omeprazole in Fasting and Fed Conditions. Clin. Drug Investig. 25, 391–399 (2005). https://doi.org/10.2165/00044011-200525060-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-200525060-00004