Abstract
Modafinil is a wake-promoting agent that is pharmacologically different from other stimulants. It has been investigated in healthy volunteers, and in individuals with clinical disorders associated with excessive sleepiness, fatigue, impaired cognition and other symptoms. This review examines the use of modafinil in clinical practice based on the results of randomized, double-blind, placebo-controlled clinical trials available in the English language in the MEDLINE database. In sleep-deprived individuals, modafinil improves mood, fatigue, sleepiness and cognition to a similar extent as caffeine but has a longer duration of action. Evidence for improved cognition in non-sleep-deprived healthy volunteers is controversial.
Modafinil improves excessive sleepiness and illness severity in all three disorders for which it has been approved by the US FDA, i.e. narcolepsy, shift-work sleep disorder and obstructive sleep apnoea with residual excessive sleepiness despite optimal use of continuous positive airway pressure (CPAP). However, its effects on safety on the job and on morbidities associated with these disorders have not been ascertained. Continued use of CPAP in obstructive sleep apnoea is essential. Modafinil does not benefit cataplexy.
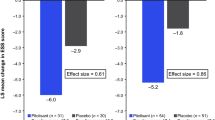
In very small, short-term trials, modafinil improved excessive sleepiness in patients with myotonic dystrophy. It was efficacious in fairly large studies of attention deficit hyperactivity disorder (ADHD) in children and adolescents, and was as efficacious as methylphenidate in a small trial, but has not been approved by the FDA, in part because of its serious dermatological toxicity. In a trial of 21 non-concurrent subjects, with 2-week treatment periods, modafinil was as effective as dexamfetamine in adult ADHD. Modafinil was helpful for depressive symptoms in bipolar disorder in a trial that excluded patients with stimulant-induced mania. A single dose of modafinil may hasten recovery from general anaesthesia after day surgery. A single dose of modafinil improved the ability of emergency room physicians to attend didactic lectures after a night shift, but did not improve their ability to drive home and caused sleep disturbances subsequently.
Modafinil had a substantial placebo effect on outcomes such as fatigue, excessive sleepiness and depression in patients with traumatic brain injury, major depressive disorder, schizophrenia, post-polio fatigue and multiple sclerosis; however, it did not provide any benefit greater than placebo.
Trials of modafinil for excessive sleepiness in Parkinson’s disease, cocaine addiction and cognition in chronic fatigue syndrome provided inconsistent results; all studies had extremely small sample sizes. Modafinil cannot be recommended for these conditions until definitive data become available.
Modafinil induces and inhibits several cytochrome P450 isoenzymes and has the potential for interacting with drugs from all classes. The modafinil dose should be reduced in the elderly and in patients with hepatic disease. Caution is needed in patients with severe renal insufficiency because of substantial increases in levels of modafinil acid. Common adverse events with modafinil include insomnia, headache, nausea, nervousness and hypertension. Decreased appetite, weight loss and serious dermatological have been reported with greater frequency in children and adolescents, probably due to the higher doses (based on bodyweight) used. Modafinil may have some abuse/addictive potential although no cases have been reported to date.


Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
National Sleep Foundation. Omnibus sleep in America poll. Washington, DC: National Sleep Foundation, 2001
Hasler G, Buysse DJ, Gamma A, et al. Excessive daytime sleepiness in young adults: a 20-year prospective community study. J Clin Psychiatry 2005; 66: 521–9
Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep 1996 May; 19(4): 318–2
Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep 1997 Apr; 20(4): 267–77
Van Dongen HP, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003 Mar 15; 26(2): 117–26
NHTSA. National Highway Traffic Safety Administration report [online]. Available from URL: http://www.nhtsa.gov/people/injury/research/drowsy_driver.html [Accessed 2006 Apr 25]
Leger D. The cost of sleep-related accidents: a report for the National Commission on Sleep Disorders Research. Sleep 1994 Feb; 17(1): 84–93
Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005 Oct 27; 437(7063): 1257–63
Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms 2006 Dec; 21(6): 482–93
Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 2008 Jun; 33(7): 1477–502
Mignot E, Nishino S, Guilleminault C, et al. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep 1994 Aug; 17: 436–7
De Séréville JE, Boer C, Rambert FA, et al. Lack of pre-synaptic dopaminergic involvement in modafinil activity in anaesthetized mice: in vivo voltammetry studies. Neuropharmacology 1994 Jun; 33: 755–61
Simon P, Hémet C, Ramassamy C, et al. Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol 1995 Dec; 5(4): 509–14
Akaoka H, Roussel B, Lin JS, et al. Effect of modafinil and amphetamine on the rat catecholaminergic neuron activity. Neurosci Lett 1991 Feb 11; 123(1): 20–2
Duteil J, Rambert FA, Pessonnier J, et al. Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil: studies with experimental animals. Eur J Pharmacol 1990 May 3; 180(1): 49–58
Lin JS, Roussel B, Akaoka H, et al. Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res 1992 Sep 25; 591(2): 319–26
Eagle DM, Tufft MR, Goodchild HL, et al. Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 2007 Jun; 192(2): 193–206
de Saint Hilaire Z, Orosco M, Rouch C, et al. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport 2001 Nov 16; 12(16): 3533–7
Wisor JP, Nishino S, Sora I, et al. Dopaminergic role in stimulant-induced wakefulness. J Neurosci 2001; 21: 1787–94
Murillo-Rodriguez E, Haro R, Palomero-Rivero M, et al. Modafinil enhances extracellular levels of dopamine in the nucleus accumbens and increases wakefulness in rats. Behav Brain Res 2007 Jan 25; 176(2): 353–7
Dopheide MM, Morgan RE, Rodvelt KR, et al. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol 2007 Jul 30; 568(1–3): 112–23
Korotkova TM, Klyuch BP, Ponomarenko AA, et al. Modafinil inhibits rat midbrain dopaminergic neurons through D2-like receptors. Neuropharmacology 2007 Feb; 52(2): 626–33
Madras BK, Xie Z, Lin Z, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther 2006 Nov; 319(2): 561–9
Mignot E, Renaud A, Nishino S, et al. Canine cataplexy is preferentially controlled by adrenergic mechanisms: evidence using monoamine selective uptake inhibitors and release enhancers. Psychopharmacology (Berl) 1993; 113(1): 76–82
Nishino S, Fruhstorfer B, Arrigoni J, et al. Further characterization of the alpha-1 receptor subtype involved in the control of cataplexy in canine narcolepsy. J Pharmacol Exp Ther 1993 Mar; 264(3): 1079–84
Gallopin T, Luppi PH, Rambert FA, et al. Effect of the wake-promoting agent modafinil on sleep-promoting neurons from the ventrolateral preoptic nucleus: an in vitro pharmacologic study. Sleep 2004 Feb 1; 27(1): 19–25
Stone EA, Cotecchia S, Lin Y, et al. Role of brain alpha 1B-adrenoceptors in modafinil-induced behavioral activity. Synapse 2002 Dec 15; 46(4): 269–70
Wisor JP, Eriksson KS. Dopaminergic-adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience 2005; 132: 1027–34
Ferraro L, Antonelli T, O’Connor WT, et al. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport 1997 Sep 8; 8(13): 2883–7
Ferraro L, Antonelli T, O’Connor WT, et al. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci Lett 1998 Sep 4; 253(2): 135–8
Ferraro L, Tanganelli S, O’Connor WT, et al. The vigilance promoting drug modafinil decreases GABA release in the medial preoptic area and in the posterior hypothalamus of the awake rat: possible involvement of the serotonergic 5-HT3 receptor. Neurosci Lett 1996 Dec 6; 220: 5–8
Ferraro L, Antonelli T, Tanganelli S, et al. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology 1999 Apr; 20(4): 346–56
Tanganelli S, Fuxe K, Ferraro L, et al. Inhibitory effects of the psychoactive drug modafinil on γ-aminobutyric acid outflow from the cerebral cortex of the awake freely moving guinea pig: possible involvement of 5-hydroxytryptamine mechanisms. Naunyn Schmiedebergs Arch Pharmacol 1992; 345: 461–5
Tanganelli S, Ferraro L, Bianchi C, et al. 6-Hydroxy-dopamine treatment counteracts the reduction of cortical GABA release produced by the vigilance promoting drug modafinil in the awake freely moving guinea-pig. Neurosci Lett 1994 Apr 25; 171(1–2): 201–4
Tanganelli S, Pérez de la Mora M, Ferraro L, et al. Modafinil and cortical gamma-aminobutyric acid outflow: modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol 1995 Jan 24; 273(1–2): 63–71
Ferraro L, Tanganelli S, O’Connor WT, et al. The vigilance promoting drug modafinil increases dopamine release in the rat nucleus accumbens via the involvement of a local GABAergic mechanism. Eur J Pharmacol 1996 Jun 13; 306: 33–9
Ferraro L, Antonelli T, O’Connor WT, et al. Modafinil: an antinarcoleptic drug with a different neurochemical profile to d-amphetamine and dopamine uptake blockers. Biol Psychiatry 1997 Dec 15; 42(12): 1181–3
Ferraro L, Fuxe K, Tanganelli S, et al. Amplification of cortical serotonin release: a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology 2000 Aug 23; 39(11): 1974–83
Ferraro L, Fuxe K, Tanganelli S, et al. Differential enhancement of dialysate serotonin levels in distinct brain regions of the awake rat by modafinil: possible relevance for wakefulness and depression. J Neurosci Res 2002 Apr 1; 68(1): 107–12
Ferraro L, Fuxe K, Agnati L, et al. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse 2005 Mar 15; 55(4): 230–41
Nishino S. The hypocretin/orexin system in health and disease. Biol Psychiatry 2003 Jul 15; 54(2): 87–95
Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999 Aug 20; 98(4): 437–51
Scammell TE, Estabrooke IV, McCarthy MT, et al. Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 2000 Nov 15; 20(22): 8620–8
Willie JT, Renthal W, Chemelli RM, et al. Modafinil more effectively induces wakefulness in orexin-null mice than in wild-type littermates. Neuroscience 2005; 130(4): 983–95
Ishizuka T, Sakamoto Y, Sakurai T, et al. Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett 2003 Mar 20; 339(2): 143–6
Ishizuka T, Murakami M, Yamatodani A. Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol 2008 Jan 14; 578(2–3): 209–15
Lagarde D, Milhaud C. Electroencephalographic effects of modafinil, an alpha-1-adrenergic psychostimulant, on the sleep of rhesus monkeys. Sleep 1990 Oct; 13: 441–8
Shelton J, Nishino S, Vaught J, et al. Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep 1995 Dec; 18: 817–26
Touret M, Sallanon-Moulin M, Jouvet M. Awakening properties of modafinil without paradoxical sleep rebound: comparative study with amphetamine in the rat. Neurosci Lett 1995 Apr 7; 189: 43–6
Edgar DM, Seidel WF. Modafinil induces wakefulness without intensifying motor activity or subsequent rebound hypersomnolence in the rat. J Pharmacol Exp Ther 1997 Nov; 283: 757–69
Panckeri KA, Schotland HM, Pack AI, et al. Modafinil decreases hypersomnolence in the English bulldog, a natural animal model of sleep-disordered breathing. Sleep 1996 Oct; 19: 626–31
Lin JS, Gervasoni D, Hou Y, et al. Effects of amphetamine and modafinil on the sleep/wake cycle during experimental hypersomnia induced by sleep deprivation in the cat. J Sleep Res 2000 Mar; 9(1): 89–96
Turner DC, Robbins TW, Clark L, et al. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology 2003; 165: 260–9
Baranski JV, Pigeau R, Dinich P, et al. Effects of modafinil on cognitive and meta-cognitive performance. Hum Psychopharmacol 2004 Jul; 19(5): 323–32
Müller U, Steffenhagen N, Regenthal R, et al. Effects of modafinil on working memory processes in humans. Psychopharmacology 2004; 177: 161–9
Makris AP, Rush CR, Frederich RC, et al. Behavioral and subjective effects of d-amphetamine and modafinil in healthy adults. Exp Clin Psychopharmacol 2007 Apr; 15(2): 123–33
Randall DC, Shneerson JM, Plaha KK, et al. Modafinil affects mood, but not cognitive function, in healthy young volunteers. Hum Psychopharmacol 2003; 18: 163–73
Randall DC, Fleck NL, Shneerson JM, et al. The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacol Biochem Behav 2004; 77: 547–55
Randall DC, Viswanath A, Bharania P, et al. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J Clin Psychopharmacol 2005; 25: 175–9
Taneja I, Haman K, Shelton RC, et al. A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol 2007 Feb; 27(1): 76–9
Lagarde D, Batejat D. Disrupted sleep-wake rhythm and performance: advantages of modafinil. Mil Psychol 1995; 7: 165–91
Pigeau R, Naitoh P, Buguet A, et al. Modafinil, d-amphetamine and placebo during 64h of sustained mental work. I, effects on mood, fatigue, cognitive performance and body temperature. J Sleep Res 1995; 4: 212–28
Stivalet P, Esquivie D, Barraud P-A, et al. Effects of modafinil on attentional processes during 60h of sleep deprivation. Hum Psychopharmacol 1998; 13: 501–7
Wesensten NJ, Belenky G, Kautz MA, et al. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology 2002; 159: 238–47
Wesensten NJ, Belenky G, Thorne DR, et al. Modafinil vs. caffeine: effects on fatigue during sleep deprivation. Aviat Space Environ Med 2004 Jun; 75(6): 520–5
Wesensten NJ, Killgore WD, Balkin TJ. Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. J Sleep Res 2005 Sep; 14(3): 255–66
Dinges DF, Arora S, Darwish M, et al. Pharmacodynamic effects on alertness of single doses of armodafinil in healthy subjects during a nocturnal period of acute sleep loss. Curr Med Res Opin 2006 Jan; 22(1): 159–67
Lagarde D, Batejat D, Van Beers P, et al. Interest of modafinil, a new psychostimulant, during a sixty-hour sleep deprivation experiment. Fundam Clin Pharmacol 1995; 9(3): 271–9
Walsh JK, Randazzo AC, Stone KL, et al. Modafinil improves alertness, vigilance, and executive function during simulated night shifts. Sleep 2004 May 1; 27(3): 434–9
Hart CL, Haney M, Vosburg SK, et al. Modafinil attenuates disruptions in cognitive performance during simulated night-shift work. Neuropsychopharmacology 2006 Jul; 31(7): 1526–36
Saletu B, Frey R, Krupka M, et al. Differential effects of a new adrenergic agonist: modafinil and D-amphetamine on sleep and early morning behaviour in young healthy volunteers. Int J Clin Pharmacol Res 1989; 9(3): 183–95
Saletu B, Frey R, Krupka M, et al. Differential effects of the new adrenergic agonist modafinil and D-amphetamine on sleep and early morning behaviour in eiderlies. Arzneimittelforschung 1989; 39(10): 1268–73
Robertson PR, Hellriegel ET. Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet 2003; 42(2): 123–7
Wong YN, Simcoe D, Hartman LN, et al. A double-blind, placebo-controlled, ascending-dose evaluation of the pharmacokinetics and tolerability of modafinil tablets in healthy male volunteers. J Clin Pharmacol 1999 Jan; 39(1): 30–40
Wong YN, King SP, Simcoe D, et al. Open-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. J Clin Pharmacol 1999 Mar; 39(3): 281–8
Moachon G, Kanmacher I, Clenet M, et al. Pharmacokinetic profile of modafinil. Drugs Today 1996; 32: 327–37
Cephalon Inc. Provigil (modafinil) tablets: prescribing information [online]. Available from URL: http://www.provigil.com/ [Accessed 2007 Feb 22]
Billiard M, Besset A, Montplaisir J, et al. Modafinil: a double-blind multicentric study. Sleep 1994 Dec; 17 (8 Suppl.): S107–12
US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Ann Neurol 1998 Jan; 43(1): 88–97
US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology 2000 Mar 14; 54(5): 1166–75
Broughton RJ, Fleming JA, George CF, et al. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology 1997; 49: 444–51
Schwartz JR, Feldman NT, Bogan RK, et al. Dosing regimen effects of modafinil for improving daytime wakefulness in patients with narcolepsy. Clin Neuropharmacol 2003 Sep–Oct; 26(5): 252–7
Schwartz JR, Nelson MT, Schwartz ER, et al. Effects of modafinil on wakefulness and executive function in patients with narcolepsy experiencing late-day sleepiness [published erratum appears in Clin Neuropharmacol 2004 May–Jun; 27 (3): 152]. Clin Neuropharmacol 2004 Mar–Apr; 27(2): 74–9
Guilleminault C, Abad VC. Obstructive sleep apnea syndromes. Med Clin North Am 2004 May; 88(3): 611–30
Kingshott RN, Vennelle M, Coleman EL, et al. Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of residual daytime excessive sleepiness in the sleep apnea/hypopneac syndrome. Am J Respir Crit Care Med 2001; 163: 918–23
Pack AI, Black JE, Schwartz JRL, et al. Modafinil as adjunct therapy for daytime sleepiness in the obstructive sleep apnea. Am J Respir Crit Care Med 2001; 164: 1675–81
Black JE, Hirshkowitz M. Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep 2005 Apr 1; 28(4): 464–71
Dinges D, Weaver TE. Effects of modafinil on sustained attention performance and quality of life in OSA patients with residual sleepiness while being treated with nCPAP. Sleep Medicine 2003; 4: 393–402
Beers TM. Flexible schedules and shift work: replacing the ‘9-to-5’ workday? Monthly Labor Rev 2000; 123: 33–40
Drake CL, Roehrs T, Richardson G, et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 2004 Dec 15; 27(8): 1453–62
Czeisler CA, Walsh JK, Roth T, et al., for the U.S. Modafinil in Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med 2005 Aug 4; 353(5): 476–86
Erman MK, Rosenberg R, for the US Modafinil Shift Work Sleep Disorder Study Group. Modafinil for excessive sleepiness associated with chronic shift work sleep disorder: effects on patient functioning and health-related quality of life. Prim Care Companion J Clin Psychiatry 2007; 9(3): 188–94
Ondo WG, Fayle R, Atassi F, et al. Modafinil for daytime somnolence in Parkinson’s disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005 Dec; 76(12): 1636–9
Hogl B, Saletu M, Brandauer E, et al. Modafinil for the treatment of daytime sleepiness in Parkinson’s disease: a double-blind, randomized, crossover, placebo-controlled trial. Sleep 2002; 25: 905–9
Adler CH, Caviness JN, Hentz JG, et al. Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Movement Disorders 2003; 18: 287–93
Laberge L, Bégin P, Montplaisir J, et al. Sleep complaints in patients with myotonic dystrophy. J Sleep Res 2004 Mar; 13(1): 95–100
MacDonald JR, Hill JD, Tarnopolsky MA. Modafinil reduces excessive somnolence and enhances mood in patients with myotonic dystrophy. Neurology 2002; 59: 1876–80
Talbot K, Stradling J, Crosby J, et al. Reduction in excess daytime sleepiness by modafinil in patients with myotonic dystrophy. Neuromuscul Disord 2003; 13: 357–64
Wintzen AR, Lammers GJ, van Dijk JG. Does modafinil enhance activity of patients with myotonic dystrophy? A double-blind placebo-controlled crossover study. J Neurol 2007 Jan; 254(1): 26–8
Jha A, Weintraub A, Allshouse A, et al. A randomized trial of modafinil for the treatment of fatigue and excessive daytime sleepiness in individuals with chronic traumatic brain injury. J Head Trauma Rehabil 2008 Jan–Feb; 23(1): 52–63
Visser SN, Lesesne CA. Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder: United States, 2003. MMWR Morb Mortal Wkly Rep 2005; 54(34): 842–7
Biederman J, Swanson JM, Wigal SB, et al. Efficacy and safety of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, flexible-dose study. Pediatrics 2005 Dec; 116(6): e777–84
Swanson JM, Greenhill LL, Lopez FA, et al. Modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder: results of a randomized, double-blind, placebo-controlled, fixed-dose study followed by abrupt discontinuation. J Clin Psychiatry 2006 Jan; 67(1): 137–47
Greenhill LL, Biederman J, Boellner SW, et al. A randomized, double-blind, placebo-controlled study of modafinil film-coated tablets in children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006 May; 45(5): 503–11
DuPaul G, Power T, Anastopoulos A, et al. ADHD rating scale-IV: home and school versions. New York: Guilford Publications, 1998
Conners CK. Conners’ parent rating scales-revised: short form (CPRS-R:S). North Tonawanda (NY): Multi-Health Systems, 1997
Gresham FM, Elliot SN. Social skills rating scale. Circle Pines (MN): American Guidance Service, 1990
Landgraf JM, Abetz L, Ware JE. The child health questionnaire (CHQ): a user’s manual. Boston (MA): Health Act, 1999
Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Psychopharmacologic Drugs Advisory Committee Meeting: March 2006 [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/06/transcripts/2006-4212T1.pdf [Accessed 2007 Feb 24]
Biederman J, Swanson JM, Wigal SB, et al. Modafinil ADHD Study Group. A comparison of once-daily and divided doses of modafinil in children with attention-deficit/hyperactivity disorder: a randomized, double-blind, and placebo-controlled study. J Clin Psychiatry 2006 May; 67(5): 727–35
Conners CK, Sitarenios G, Parker JD, et al. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998 Aug; 26(4): 257–68
Amiri S, Mohammadi MR, Mohammadi M, et al. Modafinil as a treatment for Attention-Deficit/Hyperactivity Disorder in children and adolescents: a double blind, randomized clinical trial. Prog Neuropsychopharmacol Biol Psychiatry 2008 Jan 1; 32(1): 145–9
Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med 2006 Feb; 36(2): 159–65
Moss SB, Nair R, Vallarino A, et al. Attention deficit/hyperactivity disorder in adults. Prim Care 2007 Sep; 34(3): 445–73
Taylor FB, Russo J. Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol 2000; 10(4): 311–20
Murphy KR, Barkley RA. Updated adult norms for the ADHD Behavior Checklist for Adults. ADHD Report 1996; 4: 12–13
Barkley RA, Murphy KR. Attention-deficit hyperactivity disorder: a clinical workbook. New York (NY): Guilford Press, 1998: 131–3
Golden CJ. Identification of brain disorders by the Stroop Color and Word Test. J Clin Psychol 1976; 32: 654–8
Kessler RC, Berglund P, Dernier O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593–602
Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry 2005 Jan; 66(1): 85–93
DeBattista C, Dogramji K, Menza MA, et al. Adjunct modafinil for short-term treatment of fatigue and sleepiness in patients with major depressive disorder: a preliminary double-blind, placebo-controlled study. J Clin Psychiatry 2003; 64: 1057–64
Dunlop BW, Crits-Christoph P, Evans DL, et al. Coadministration of modafinil and a selective serotonin reuptake inhibitor from the initiation of treatment of major depressive disorder with fatigue and sleepiness: a double-blind, placebo-controlled study. J Clin Psychopharmacol 2007 Dec; 27(6): 614–9
Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 2007 Aug; 164(8): 1242–9
Rush AJ, Giles DE, Schlesser MA. The Inventory of Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res 1986; 18: 65–87
Turner DC, Clark L, Pomarol-Clotet E, et al. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology 2004 Jul; 29(7): 1363–73
Sevy S, Rosenthal MH, Alvir J, et al. Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry 2005 Jul; 66(7): 839–43
Spence SA, Green RD, Wilkinson ID, et al. Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry 2005 Jul; 187: 55–61
Pierre JM, Peloian JH, Wirshing DA, et al. A randomized, double-blind, placebo-controlled trial of modafinil for negative symptoms in schizophrenia. J Clin Psychiatry 2007 May; 68(5): 705–10
Cambridge Neuropsychological Test Automated Battery (CANTAB®) [online]. Available from URL: http://www.cantab.com [Accessed 2007 Nov 1]
Callicott JH, Ramsey NF, Tallent K, et al. Functional magnetic resonance brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 1998; 18: 186–96
Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull 1988; 24: 97–9
Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Br J Psychiatry 1989; 155 Suppl. 7: 53–8
Guy W, editor. ECDEU assessment manual for psychopharmacology. US Dept Health, Education and Welfare publication (ADM): Rockville (MD): National Institute of Mental Health, 1976: 76–338
Simpson GM, Lee JH, Zoubok B, et al. A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979; 64: 171–9
Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212: 11–9
Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II, contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res 1989 Jul; 29(1): 65–85
Cornblatt BA, Obuchowski M, Roberts S, et al. Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol 1999; 11: 487–508
Gold JM, Carpenter C, Randolph C, et al. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 1997 Feb; 54(2): 159–65
Hershey T, Craft S, Glauser TA, et al. Short-term and long-term memory in early temporal lobe dysfunction. Neuropsychology 1998 Jan; 12(1): 52–64
Lezak MD. Neuropsychological assessment. Third edition. New York (NY): Oxford University Press, 1995: 438–45, 544–50
Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry 1982 Jul; 39(7): 784–8
Dackis CA, Kampman KM, Lynch KG, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005 Jan; 30(1): 205–11
Hart CL, Haney M, Vosburg SK, et al. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology 2008 Mar; 33(4): 761–8
Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: psychosocial and biological medthods. Totowa (NJ): Humana Press, 1992: 41–72
Kampman KM, Volpicelli JR, McGinnis DE, et al. Reliability and validity of the cocaine selective severity assessment. Addict Behav 1998; 23: 449–61
Somoza E, Dyrenforth S, Goldsmith J, et al. In search of a universal drug craving scale. Annual Meeting of the American Psychiatric Association; 1995 May 20–25; Miami (FL)
Tiffany ST, Singleton E, Haertzen CA, et al. The development of a cocaine craving questionnaire. Drug Alcohol Depend 1993; 34: 19–28
Randall DC, Cafferty FH, Shneerson JM, et al. Chronic treatment with modafinil may not be beneficial in patients with chronic fatigue syndrome. J Psychopharmacol 2005 Nov; 19(6): 647–60
Chan KM, Strohschein FJ, Rydz D, et al. Randomized controlled trial of modafinil for the treatment of fatigue in post-polio syndrome. Muscle Nerve 2006 Jan; 33(1): 138–41
Vasconcelos OM, Prokhorenko OA, Salajegheh MK, et al. Modafinil for treatment of fatigue in post-polio syndrome: a randomized controlled trial. Neurology 2007 May 15; 68(20): 1680–6
Stankoff B, Waubant E, Confavreux C, et al. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. French Modafinil Study Group. Neurology 2005 Apr 12; 64(7): 1139–43
Larijani GE, Goldberg ME, Hojat M, et al. Modafinil improves recovery after general anesthesia. Anesth Analg 2004 Apr; 98(4): 976–81
Gill M, Haerich P, Westcott K, et al. Cognitive performance following modafinil versus placebo in sleep-deprived emergency physicians: a double-blind randomized crossover study. Acad Emerg Med 2006 Feb; 13(2): 158–65
Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol 2000 Mar; 14(1): 53–60
Martin WR, Sloan JW, Sapira JD, et al. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 1971 Mar–Apr; 12(2): 245–58
Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends Pharmacol Sci 2004 Apr; 25(4): 193–200
Robertson P, DeCory HH, Madan A, et al. In vitro inhibition and induction of human hepatic cytochrome P450 enzymes by modafinil. Drug Metab Dispos 2000 Jun; 28(6): 664–71
Robertson Jr P, Hellriegel ET, Arora S, et al. Effect of modafinil at steady state on the single-dose pharmacokinetic profile of warfarin in healthy volunteers. J Clin Pharmacol 2002 Feb; 42(2): 205–14
Robertson Jr P, Hellriegel ET, Arora S, et al. Effect of modafinil on the pharmacokinetics of ethinyl estradiol and triazolam in healthy volunteers. Clin Pharmacol Ther 2002 Jan; 71(1): 46–56
FDA. Provigil® (modafinil) tablets (C-IV) [online]. Available from URL: http://www.fda.gov/cder/foi/label/2004/20717sel008_provigil_lbl.pdf [Accessed 2007 Mar 3]
Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med 2005 May 26; 352(21): 2211–21
Bernard S, Neville KA, Nguyen AT, et al. Interethnic differences in genetic polymorphisms of CYP2D6 in the US population: clinical implications. Oncologist 2006 Feb; 11(2): 126–35
Mariani JJ, Hart CL. Psychosis associated with modafinil and shift work [letter]. Am J Psychiatry 2005 Oct; 162(10): 1983
Narendran R, Young CM, Valenti AM, et al. Is psychosis exacerbated by modafinil? Arch Gen Psychiatry 2002 Mar; 59(3): 292–3
Sabatine MS, Poh KK, Mega JL, et al. Case records of the Massachusetts General Hospital: case 36-2007-a 31-year-old woman with rash, fever, and hypotension. N Engl J Med 2007 Nov 22; 357(21): 2167
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R. Approved and Investigational Uses of Modafinil. Drugs 68, 1803–1839 (2008). https://doi.org/10.2165/00003495-200868130-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200868130-00003