Abstract
Background: Prescribing drugs to pregnant women requires the balancing of benefits and risks. Only a small proportion of drugs are known to be harmful to the fetus, but for the vast majority of drugs little evidence of fetal safety exists.
Aim: To determine the prescription pattern of potentially and clearly harmful prescription drugs during pregnancy with reference to drug safety categorisation, and to define the drug groups primarily responsible for multiple drug use during pregnancy.
Study design: A retrospective, register-based cohort study.
Methods: Linkage of three nationwide registers in Finland. Data collection included prescription drugs purchased during the preconception period and each trimester in the pregnant cohort, and the corresponding time periods in the non-pregnant controls.
The pregnancy safety categorisation was determined for each drug (Anatomic Therapeutic Chemical [ATC] code) by using the Swedish classification of approved medicinal products (Farmaceutiska Specialiteter i Sverige [FASS]) and if not available, the corresponding Australian (Australian Drug Evaluation Committee [ADEC]) or US categorisation (FDA).
Groups studied: Women applying for maternity support (maternal grants) during the year 1999 (n = 43 470) plus non-pregnant control women matched by age and hospital district (n = 43 470).
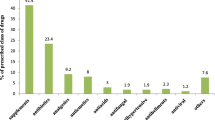
Results: In the pregnant cohort, 20.4% of women purchased at least one drug classified as potentially harmful during pregnancy, and 3.4% purchased at least one drug classified as clearly harmful. A significant decline occurred in the number of pregnant women purchasing potentially and clearly harmful drugs during the first trimester when compared with the preconception period, and the decline continued from the first to the second trimester. In the pregnant cohort, 107 (0.2%) women purchased at least ten different drugs during pregnancy. The drugs most commonly purchased in this group were topical corticosteroids and nasal preparations.
Conclusion: The use of hazardous prescription drugs declines during pregnancy but prescriptions of known teratogens and the relatively frequent practice of polypharmacy in epilepsy place emphasis on the need for careful pre-pregnancy counselling. However, drug safety classifications give a very crude estimation of risk and should only be used as general guidelines when planning treatment. Risk assessment must always be made on an individual basis, and pregnant women with illnesses requiring treatment must be treated adequately.







Similar content being viewed by others
References
Kalter H. Environmental causes of congenital malformations in humans and how they were established. Neurotoxicol Teratol 2003; 25: 131–282
Webster WS, Freeman JA. Prescription drugs and pregnancy. Expert Opin Pharmacother 2003; 4: 949–61
Addis A, Sharabi S, Bonati M. Risk classification systems for drug use during pregnancy: are they a reliable source of information? Drug Saf 2000; 23(3): 245–53
Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 5th ed. Baltimore (MD): Lippincott Williams & Wilkins, 1998
Doering PL, Boothby LA, Pharm D, et al. Review of pregnancy labeling of prescription drugs: is the current system adequate to inform of risk? Am J Obstet Gynecol 2002; 187: 333–9
Sannerstedt R, Lundborg P, Danielsson BR, et al. Drugs during pregnancy: an issue of risk classification and information to prescribers. Drug Saf 1996; 14(2): 69–77
FASS. Classification of medical products for use during pregnancy and lactation. The Swedish system. Stockholm: LINFO, Drug Information Ltd, 1993
Australian Drug Evaluation Committee. Medicines in pregnancy: an Australian categorisation of risk of drug use in pregnancy. 3rd edition. Canberra; Australian Government Publishing Service, 1996
Malm H, Martikainen J, Klaukka T, et al. Prescription drugs during pregnancy and lactation: a Finnish register-based study. Eur J Clin Pharmacol 2003; 59: 127–33
De Vigan C, De Walle HC, Cordier S, et al. Therapeutic drug use during pregnancy: a comparison in four European countries. J Clin Epidemiol 1999; 52: 977–82
Donati S, Baglio G, Spinelli A, et al. Drug use in pregnancy among Italian women. Eur J Clin Pharmacol 2000; 56: 323–8
Olesen C, Hald Steffensen F, Nielsen GL, et al. Drug use in first pregnancy and lactation: a population-based survey among Danish women. Eur J Clin Pharmacol 1999; 55: 139–44
Lacroix I, Damase-Michel C, Lapeyre-Mestre M, et al. Prescription of drugs during pregnancy in France. Lancet 2000; 356: 1735–6
Headley J, Northstone K, Simmons H, et al. Medication use during pregnancy: data from the Avon Longituninal Study of Parents and Children. Eur J Clin Pharmacol 2004; 60: 335–61
Olesen C, Sondergaard C, Thrane N, et al. Do pregnant women report use of dispensed medications? Epidemiology 2001; 12: 497–501
Olesen C, Sorensen HT, de Jong-van den Berg L, et al. Prescribing during pregnancy and lactation with reference to the Swedish classification system: a population-based study among Danish women. The Euromap Group. Acta Obstet Gynecol Scand 1999; 78: 686–92
Schirm E, Meijer WM, Tobi H, et al. Drug use by pregnant women and comparable non-pregnant women in the Netherlands with reference to the Australian classification system. Eur J Obstet Gyn 2004; 114: 182–8
Källen B, Rydhstrom H, Aberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstet Gynecol 1999; 93: 392–5
Bianca S. Drug use during pregnancy: are risk classifications more dangerous than the drugs? [letter]. Lancet 2003; 362: 329
FASS. Farmaceutiska Specialiteter i Sverige. Lakemedel i Sverige. LINFO. Oslo; Elanders Publishing, 1999
Christensen B. Which antibiotics are appropriate for treating bacteriuria in pregnancy? J Antimicrob Chemother 2000; 46Suppl. 1: 29–34
Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med 1998; 338: 1128–37
Nulman I, Berkovitch M, Klein J, et al. Steady-state pharmacokinetics of isotretinoin and its 4-oxo metabolite: implications for fetal safety. J Clin Pharmacol 1998; 38: 926–30
Balasubramaniam J. Nimesulide and neonatal failure [letter]. Lancet 2000; 355: 575
Pennell PB. The importance of monotherapy during pregnancy. Neurology 2003; 60Suppl. 4: 31–8
Acknowledgments
The authors thank Ms Hilkka Ruuska at The Social Insurance Institution, Helsinki, for her skilled technical assistance in linking and processing the databases. No sources of funding were used to assist in the preparation of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malm, H., Martikainen, J., Klaukka, T. et al. Prescription of Hazardous Drugs During Pregnancy. Drug-Safety 27, 899–908 (2004). https://doi.org/10.2165/00002018-200427120-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200427120-00006