Abstract
Rotaviruses are the most common cause of severe gastroenteritis in children. By 5 years of age virtually every child worldwide will have experienced at least one rotavirus infection. This leads to an enormous disease burden, where every minute a child dies because of rotavirus infection and another four are hospitalized, at an annual societal cost in 2007 of $US2 billion. Most of the annual 527 000 deaths are in malnourished infants living in rural regions of low and middle income countries. In contrast, most measurable costs arise from medical expenses and lost parental wages in high income countries.
Vaccines are the only public health prevention strategy likely to control rotavirus disease. They were developed to mimic the immunity following natural rotavirus infection that confers protection against severe gastroenteritis and consequently reduces the risk of primary healthcare utilization, hospitalization and death. The two currently licensed vaccines — one a single human strain rotavirus vaccine, the other a multiple strain human-bovine pentavalent reassortant rotavirus vaccine — are administered to infants in a two- or three-dose course, respectively, with the first dose given at 6–14 weeks of age. In various settings they are safe, immunogenic and efficacious against many different rotavirus genotypes. In high and middle income countries, rotavirus vaccines confer 85–100% protection against severe disease, while in low income regions of Africa and Asia, protection is less, at 46–77%. Despite this reduced efficacy in low income countries, the high burden of diarrheal disease in these regions means that proportionately more severe cases are prevented by vaccination than elsewhere. Post-licensure effectiveness studies show that rotavirus vaccines not only reduce rotavirus activity in infancy but they also decrease rates of rotavirus diarrhea in older and unimmunized children. A successful rotavirus vaccination program will rely upon sustained vaccine efficacy against diverse and evolving rotavirus strains and efficient vaccine delivery systems. The potential introduction of rotavirus vaccines into the world’s poorest countries with the greatest rates of rotavirus-related mortality is expected to be very cost effective, while rotavirus vaccines should also be cost effective by international standards when incorporated into developed countries immunization schedules. Nonetheless, cost effectiveness in each country still depends largely on the local rotavirus mortality rate and the price of the vaccine in relation to the per capita gross domestic product.
Similar content being viewed by others
1. Introduction
Rotaviruses are the most common cause of severe gastroenteritis in infants and young children worldwide, resulting each year in more than half a million deaths globally in this age group from severe dehydration, and electrolyte and acid-base disturbance.[1] By 5 years of age almost all children will have experienced one or more rotavirus infections irrespective of where they live or their socioeconomic status.[2,3] This means that improvements in housing, water supply, sanitation, personal hygiene, food quality, nutrition, and maternal education are unlikely to reduce the overall incidence of rotavirus infections. Consequently, vaccines are seen as the only effective public health intervention capable of controlling rotavirus disease.
This review provides an update on the global health and economic burden of rotavirus disease. It summarizes the relevant principles of rotavirus vaccine development and presents efficacy and effectiveness data from both developed and developing countries for the two currently licensed vaccines. We searched MEDLINE, PubMed and the Cochrane Collaboration using ‘rotavirus’ and ‘rotavirus vaccines’ as both medical subject headings and keywords, concentrating mainly upon articles published since 2005. The search was supplemented by our personal files, conference attendance, and web-based searches for international conferences where rotavirus papers were presented.
2. Disease Burden
2.1 Global
Acute gastroenteritis is second only to pneumonia as the leading global cause of childhood mortality and morbidity, accounting for 15% of all deaths in children <5 years of age.[4] Annually, rotavirus infections alone cause an estimated 111 million episodes of gastroenteritis for which healthcare is not sought, and are responsible for 25 million clinic visits, more than 2 million hospital admissions, and 527 000 deaths in children <5 years of age.[2,5] Overall, 29% of all childhood deaths from diarrhea are due to rotaviruses in this age group.[5] However, these mortality figures, which are based upon a systematic review of the published literature may underestimate the actual burden of disease, as results from global surveillance networks that use standardized testing protocols suggest as many as 39% of diarrhea-related deaths are associated with rotaviruses.[1,6]
2.2 Low and Middle Income Countries
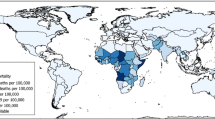
Approximately 99% of rotavirus deaths occur in low and middle income countries (figure 1),[7] and more than half of these are from just six countries: India, Nigeria, Congo, Ethiopia, China, and Pakistan.[5] Most deaths occur in malnourished infants living in socioeconomically disadvantaged rural regions in the low income countries of Africa and Asia, where access to healthcare is poor and where by 5 years of age more than one in 240 children will die from a rotavirus infection.[2,5,8]
Rotavirus mortality rates (reproduced from Danchin and Bines,[7] with permission. Copyright © 2009 Massachusetts Medical Society. All rights reserved).
In India alone, rotaviruses cause more than 120 000 deaths annually, 450 000 hospitalizations, 5 million clinic attendances, and 25 million diarrheal episodes in children <5 years of age.[1,9,10] The Asian Rotavirus Surveillance Network, which encompasses low, middle and high income countries, estimates that 45% of diarrhea-related hospital admissions within its region are due to rotaviruses.[11] The disease burden is similarly high in sub-Saharan Africa, where an estimated 300 000 children aged <5 years die each year from rotavirus gastroenteritis.[12] As noted elsewhere,[2] the proportion of gastroenteritis cases due to rotaviruses in young African children increases with disease severity, ranging from 4% in community-based studies, to 23% in those attending outpatient clinics, and to 34% in those requiring admission to hospital.
In contrast to the low income countries of Africa and Asia, the emerging middle income nations of Latin America have lower rotavirus mortality rates. Nevertheless, each year in Latin America, rotaviruses cause 15 000 deaths, and result in 75 000 hospitalizations, 2 million outpatient clinic visits, and an estimated 10 million episodes of gastroenteritis.[13] Recent rotavirus sentinel surveillance results from Latin America show that rotaviruses were detected in 42% of children admitted to hospital with severe diarrhea.[14]
2.3 High Income Countries
While deaths from rotavirus are rare in the high income countries of North America, Europe, East Asia, and Australasia, the incidence of disease in young children is similar to that of low and middle income countries, imposing a considerable burden upon their health systems and economies. In the pre-rotavirus vaccine era, rotavirus gastroenteritis resulted in 220 000 annual hospital admissions, 1.8 million healthcare visits, and 7.1 million episodes of diarrhea among children living in high income countries.[1,2,15] By 5 years of age, approximately one in 50 children from these wealthy countries will have been hospitalized following a rotavirus infection. Prior to the introduction of rotavirus vaccines into the US, it was estimated that rotavirus led to more than 2.7 million cases of gastroenteritis, 780 000 clinic visits, 164 000 emergency department attendances, as many as 117 000 hospitalizations, and almost 40 deaths each year.[16–19] Meanwhile, in the EU, rotaviruses are responsible every year for 230 deaths, nearly 90 000 hospital admissions, 700 000 outpatient consultations, and 2.8 million diarrheal illnesses.[20] The annual society-related costs from rotavirus infection and its management were almost $US900 million in the US in 2004 and €550 million in the EU in 2002.[18,21] In comparison, total societal costs of just $US423 million in 2007 were reported for all lower and middle income countries.[22]
For every child admitted to hospital because of rotavirus gastroenteritis in a high income country, approximately ten will be seen in primary care and between 30 and 40 will be managed at home without seeking medical advice.[23–25] While 20–30% of children <5 years of age presenting to their family practitioner with acute diarrhea will have rotavirus in their stools, this proportion increases to 40–60% for those more severely ill and managed in an emergency department or hospital setting.[23,26–30]
2.4 Healthcare-Associated Infections
Rotaviruses are an important cause of healthcare-associated infections in infants and young children. They are responsible for 31–87% of all healthcare-associated episodes of gastroenteritis, about one-third of which are judged as being severe, and these episodes account for 7.5–32% of all children with rotavirus diarrhea in hospital.[29,31–33] The incidence of healthcare-associated rotavirus infections is between 0.3 and 4.8 per 1000 hospital-days.[29,31,34] This means that until the recent introduction of rotavirus vaccines, in the US alone there were 15 000–20 000 children annually developing a symptomatic rotavirus infection while being cared for in hospital.[35] The rotavirus strains in hospital appear to be introduced from the community,[32,36] possibly by children with prolonged excretion of rotaviruses following severe diarrhea.[37] Asymptomatic healthcare staff may also play a role,[38] since many cases of hospital-associated rotavirus infections occur in young children who have been in hospital for more than a week and are nursed in areas of the hospital that do not normally care for children with community-acquired gastroenteritis.[32]
A study from Israel showed that hospital-acquired rotavirus infection increased hospital stay by a median of 3 days.[39] In Europe these infections result in additional costs of €2500 per case or €50 million per year (2004 values).[33]
2.5 Limitations of Disease Burden Estimates
When interpreting rotavirus disease burden estimates it is important to consider the limitations associated with rotavirus surveillance and data collection. As rotavirus testing and coding are not performed routinely, national hospital discharge coding and insurance claim data record only a fraction of rotavirus-related hospitalizations and healthcare encounters. A recent prospective, active surveillance study in the US reported that only 11% of children with laboratory-confirmed rotavirus infection who presented to hospital and outpatient clinics received a rotavirus-specific discharge code, while 61% of children assigned an acute gastroenteritis code were misclassified as they had positive stool samples for rotavirus.[19] Indirect methods attempt to address this problem. An example is the winter residual method, which calculates the excess diarrheal episodes during winter compared with summer and assumes the difference is solely from cases of rotavirus.[16,17,26]
Active surveillance for rotavirus is preferred, but incomplete collection and testing of stools from children with acute diarrhea and extrapolation of findings from a few centers to a national population may not provide representative data.[19,27–30] In addition, much of the active rotavirus surveillance is hospital based.[6] Hospitalization practices vary between and within countries, being dependent upon access to primary healthcare, referral patterns, healthcare-seeking behavior, and cultural attitudes of the local population, disease management, and hospital admission policies.[34,40] As the proportion of cases with rotavirus infection increases with disease severity, this should be considered when comparing sites since the proportion of rotavirus cases may be less than expected in centers where a low threshold for hospital admission exists. Similarly, if other enteric pathogens are more prevalent in the community the increased denominator will decrease the proportion of rotavirus cases even though the number of rotavirus hospitalizations is unchanged.[6] Hospital-based data can also underestimate the true rates of rotavirus-related mortality and severe disease in areas with poor access to healthcare.[8]
3. Clinical Features and Epidemiology
3.1 Clinical Illness
Rotavirus infections can range from a subclinical illness to mild diarrhea to severe gastroenteritis with dehydration. After an incubation period of 2–4 days, symptoms typically begin abruptly with fever and vomiting, followed by watery diarrhea lasting for 3–8 days.[41] The vomiting can be intense and may limit attempts at oral rehydration. Compared with other viral enteropathogens, rotaviruses are more likely to result in symptomatic illness and to lead to signs of dehydration.[42,43] Most children following a severe rotavirus diarrheal episode will shed rotavirus for 1–3 weeks. However, approximately one in five will continue to shed the virus for 4–8 weeks, especially if the children are experiencing mild or intermittent gastrointestinal symptoms.[37]
Rotavirus disease is most common and severe in children between 3 and 36 months of age. Multiple infections can occur throughout life, although cumulative immunity means that these episodes are usually mild or asymptomatic in older children and adults.[44] In contrast, clinical illness is uncommon in neonates.[45] Immaturity of the neonatal gut, maternal antibodies and the reduced virulence properties of unique rotavirus strains capable of replicating in the neonatal gut may play a role in the subclinical infection seen in neonates. Meanwhile, rotaviruses are associated with prolonged illness and stool shedding in infants with severely compromised T-cell immunity, such as those with severe combined immunodeficiency, and in children and adults immediately following bone marrow transplantation.[46] Recently, three infants with severe combined immunodeficiency were reported to develop severe diarrhea and prolonged shedding of vaccine virus after rotavirus vaccination.[47] Of interest, children with asymptomatic or mildly symptomatic HIV infection and less impaired immunity seem not to be at increased risk of severe rotavirus disease, although they too may shed rotavirus particles for longer periods than other children.[48]
Despite rotaviruses being associated with elevated serum aspartate aminotransferase levels[49] and rare cases of encephalopathy where rotavirus antigen and RNA are detected in the cerebrospinal fluid,[50,51] rotaviruses were traditionally believed to infect only mature epithelial cells lining the intestinal tract. However, rotavirus antigen and RNA have been detected recently in the serum of children hospitalized with severe gastroenteritis.[52,53] The highest levels of antigenemia were detected early in the illness and in those with high fever. Nonetheless, the clinical significance of extraintestinal rotavirus infection other than for fever associated with viremia or antigenemia is unknown, and its impact upon the development of natural and vaccine-induced immunity is yet to be determined.[51]
3.2 Transmission
Rotaviruses are highly contagious. The infectious dose is low[54] and the virus is shed in large quantities, as much as 1011 particles per gram of stool, both before the onset of symptoms and for several weeks afterwards.[37,55] Furthermore, the virus survives on dry surfaces for as long as 10 days and on human hands for up to 4 hours.[56] Transmission to susceptible individuals is mainly by the fecal-oral route from direct contact with rotavirus cases, including children and adults with subclinical illness, and after contact with contaminated fomites, food and water, and environmental surfaces.[37,38,56–59] Despite these formidable obstacles, improving hand hygiene within hospitals can decrease healthcare-associated rotavirus infections.[60] It has also been suggested that aerosol transmission might be important; simultaneous outbreaks have occurred in isolated communities on Native American reservations and in Aboriginal infants in Central Australia.[61,62]
3.3 Epidemiology
Rotavirus disease severity is age dependent. The first infection, while often mild or asymptomatic, is usually the most significant and can be severe. A 13-year study from Melbourne, Australia, found rotaviruses to be predominant in children aged between 6 and 36 months, and for rotavirus-associated hospital admissions to peak in the 12- to 24-month age group.[63] A community-based study followed 200 Mexican children from birth to 2 years of age by collecting weekly stool samples.[64] In this cohort, the cumulative proportions experiencing a primary rotavirus infection were 34%, 67%, and 96% by the ages of 6, 12, and 24 months, respectively. Multiple infections were also common; by 2 years of age, 69% of the cohort had recorded at least two rotavirus infections, 42% experienced three infections and 13% had been infected five times.
In countries and regions with a temperate climate, rotaviruses display a seasonal pattern, with a distinct peak encompassing winter and spring months when both the ambient temperature and humidity are low.[46,61,65] These annual epidemics coincide with the peak activity of respiratory viruses, which places additional pressure on primary and hospital healthcare services. Such marked seasonality is not seen in the tropics, although the greatest activity is still observed during the cooler and drier months of the year.[62] When minimal seasonality exists, rotaviruses circulate at relatively high levels all year round, resulting in children being exposed at a younger age and experiencing more severe illness than children living in regions where circulation of the virus is more uneven.[66] Under these circumstances, as many as 80% of infants will experience their first rotavirus infection before 12 months of age. In contrast, in temperate climates, infants born during the summer months are at greatest risk of rotavirus infection during infancy.[67] However, climatic factors alone may not explain seasonal variations in rotavirus transmission. Crowding, increased birth rates, poor water quality, and changes in host behavior for example spending more time indoors, may also be important.[65,66,68]
Preterm birth, low birthweight, socioeconomic disadvantage, malnutrition, co-infection with bacterial enteropathogens, and impaired immunity are risk factors for severe rotavirus gastroenteritis requiring primary healthcare attendance or admission to hospital.[69–72] Indigenous populations in high income countries possess many of these risk factors, and, as might be expected, have high rates of severe rotavirus disease.[73] For example, in the Northern Territory of Australia, notification rates in Aboriginal children under 5 years of age for rotavirus gastroenteritis (2.75 per 100 per year) were almost three times that of non-indigenous children from the same region.[74] In neighboring Queensland, indigenous children were at higher risk of being hospitalized due to rotavirus infection, and when this occurred, it was at an earlier age, peaking in those <12 months of age, and for longer periods than in non-indigenous contemporaries.[75] Breast feeding during the first 6 months of life appears protective for all infants, but this protection is incomplete and disappears shortly after weaning or when a mixed diet is introduced.[69,71,76]
4. Scientific Foundation of Rotavirus Vaccines
4.1 Rotavirus Biology
Rotaviruses belong to the Reoviridae family and the rotavirus genus.[46] They are non-enveloped, double-stranded RNA viruses containing 11 segments of genomic RNA surrounded by a triple layered capsid. The viral genome encodes six structural (VP1–VP4, VP6, and VP7) and six non-structural (NSP1-NSP6) proteins. The most abundant viral protein is VP6, which bears the group-specific antigenic determinants and forms the middle capsid layer, while the outer layer proteins, VP7 and VP4, act as independent neutralizing antigens (figure 2). NSP4 is also important; it is antigenic, allowing categorization into different genogroups, and is believed also to act as an enterotoxin capable of causing diarrhea.[46]
Schematic rotavirus structure (adapted from The Lancet, Cunliffe et al.,[77] Copyright 2002, with permission from Elsevier). dsRNA = double-stranded ribonucleic acid.
Within the rotavirus genus are seven antigenically distinct serogroups (A to G), but group A rotaviruses are the predominant cause of illness in humans and animals. Gene expression of the two major outer capsid surface proteins, VP7 (G type) glycoprotein and the protease cleaved attachment protein VP4 (P type), form the basis for a binary classification system. Both proteins have epitopes that induce serotype-specific and serotype cross-reacting neutralizing antibodies, which are likely to play an important role in protective immunity.[78]
Segregation of VP7 and VP4 genes occurs independently. To date, 23 VP7 and 31 VP4 genotypes have been identified, of which 12 of each type are found in humans.[79] Globally, in humans G1, G2, G3, G4, and G9 are the most prevalent VP7 genotypes, P[8], P[4], and P[6] are the most common VP4 genotypes, while G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] combinations make up 70–90% of circulating rotavirus strains.[80–82] Overall, G1P[8] predominates and is responsible for almost 70% of cases worldwide, followed by G2P[4], which accounts for approximately 12% of circulating strains globally, although this varies with time and geography.[80] In Africa, for example, G1P[8] strains are detected in <30% of children with rotavirus gastroenteritis.[12,80] In contrast, although G8P[6] is the fourth most common genotype detected in sub-Saharan Africa, elsewhere it is detected rarely.[12,79] Strain diversity is greatest in Africa and Asia, where mixed infections with distinct rotavirus strains and close proximity to certain domestic animals shedding rotaviruses such as pigs and cattle are common.[12,79] In addition to regional differences, major changes in one or more dominant circulating strain genotypes can occur from one season to another.[79–85] Rotavirus genomic diversity arises from several mechanisms, including sequential point mutations, genomic reassortment during mixed infections, genomic rearrangement within gene segments, and interspecies transmission.[77] Continuing rotavirus evolution may have important implications for vaccine development as newly emerging global strains, such as the porcine origin G12P[6], can give rise to novel human strains, which fail to share neutralizing antigens with the current vaccines.[86,87]
4.2 Protective Immunity
Individuals can experience multiple rotavirus infections throughout their life.[44] However, cumulative immunity following each infection provides increasing protection against subsequent infection and illness. A longitudinal birth cohort study in Mexican infants found that the degree of protection was greatest against severe disease.[64] The initial natural rotavirus infection provided 38% protection against asymptomatic infection, 73% protection against mild diarrhea, and 87% protection against moderate to severe disease. After a second episode the degree of protection increased to 60% for asymptomatic infection, and 83% for mild rotavirus diarrheal illness, and complete protection was provided against infection resulting in moderate to severe symptoms. A third infection was needed, however, for complete protection against mild disease. Overall, subsequent infections were less severe and more likely to be caused by another rotavirus serotype. Asymptomatic primary infections appeared to provide similar protection to those associated with symptoms of any severity. Similar findings were observed in Melbourne, Australia, following asymptomatic infection of newborn babies by unique neonatal strains. These babies were subsequently protected against severe disease, but not infection, from circulating community rotavirus strains that did not share the same G and P types.[88]
Despite the protective effects of repeated rotavirus infections in early childhood being well documented, the underlying immune mechanisms are not fully understood, and there is still no completely reliable correlate of protection. In children following rotavirus infection, IgA, IgG and neutralizing antibodies in serum, as well as fecal IgA, are associated with protection.[89–91] After a primary infection, the serum neutralizing antibody response to VP7 is predominantly homotypic or G type-specific, while responses to VP4 proteins are cross-reactive or heterotypic.[92,93] Upon reinfection there is a broadening of the neutralizing response to other G serotypes.[93] Nevertheless, challenge studies in adult volunteers with G1P[8] strains have shown that protection is most closely associated with serum IgG antibodies directed against homotypic VP7 and VP4 neutralizing antigens.[94] However, the association between rotavirus antibody levels and protection is incomplete, suggesting that other factors or target antigens, such as VP6 or NSP4, are also playing a role in protective immunity.
The relationship between vaccine-induced immunity and protection is less evident than observed with natural infection.[95] Even though the proportion of individuals undergoing seroconversion following vaccination varies between vaccines, each vaccine provides a high degree of protection against severe disease.
5. Rotavirus Vaccines
Rotavirus vaccines aim to mimic the protection provided by natural rotavirus infections by protecting against moderate to severe disease. The aims of rotavirus vaccine programs are to prevent deaths and hospitalization from severe gastroenteritis, to decrease the number of medical consultations for acute rotavirus diarrhea in children, and to reduce the economic burden of rotavirus disease on the healthcare system and society.
The first licensed rotavirus vaccine, RotaShield®, was developed by reassortant technology where single human rotavirus VP7 genes were substituted into the backbone of the remaining ten rhesus monkey rotavirus genes. Although this vaccine was highly effective at preventing severe gastroenteritis,[96] it was withdrawn after 9 months and distribution of almost 1 million doses when an association with acute intussusception, a rare disorder leading to potentially life-threatening bowel obstruction, was first recognized.[97] The risk estimate of intussusception was about 1 in 10 000, and cases clustered mostly within 3–14 days of the first dose. The risk of intussusception appeared highest in those receiving their first dose after 3 months of age.[98] The cause of the association between RotaShield® and intussusception remains unexplained. However, it has meant that subsequent candidate rotavirus vaccines have to undergo large field trials, not only to prove efficacy in the absence of a reliable correlate of protection, but also to demonstrate safety against at least a 1 in 10 000 risk of intussusception. As an added precaution, strict vaccination schedules have been implemented, with the first dose administered at age 6–14 weeks, and no ‘catch-up’ campaigns are allowed. Moreover, several countries have introduced post-licensure monitoring for intussusception.
The two currently licensed vaccines (see sections 5.1 and 5.2) were developed separately using different biological principles to achieve protection against a broad range of circulating rotavirus serotypes. However, they cannot be compared directly, as the main phase III trials were conducted in different settings and populations, different case definitions and clinical scoring systems were used to assess severity, and different techniques were employed to detect vaccine virus shedding.[99]
5.1 Human-Bovine Pentavalent Reassortant Rotavirus Vaccine (PRV)
5.1.1 Profile
Pentavalent rotavirus vaccine (PRV; RotaTeq®, Merck & Co., West Point, PA, USA) is a live oral vaccine that is stored at 2–8°C and contains five human-bovine reassortant rotavirus strains suspended in a buffered liquid to protect them against gastric acid.[100] These animal-derived strains replicate less efficiently than human strains within the human gut. PRV is administered as a three-dose oral series, beginning at 6–14 weeks of age, followed at 4- to 10-week intervals by subsequent doses, with the last dose administered before 8 months of age.[100]
Each of the five reassortant strains consists of a bovine core. Four of the reassortant rotaviruses express one of the outer capsid proteins (G1, G2, G3, or G4) from the human rotavirus parent strains and the attachment protein (P[5]) from the bovine rotavirus parent strain. The fifth reassortant virus expresses the attachment protein (P[8]) from the human rotavirus parent strain and the outer capsid protein (G6) from the bovine rotavirus parent strain.[100,101] The aim of including multiple strains is to maximize protection by promoting homotypic immunity against the most common human rotavirus VP7 G types and the predominant VP4 P type circulating worldwide.[102] Nevertheless, despite including these multiple strains and inducing serum IgA seroconversion in >90% of healthy Finnish, North American and Korean infants, individual PRV serotype-specific conversion rates are disappointing, ranging from 15–35% for G2 to as high as 55–86% for G1.[103–106] Furthermore, within individuals, vaccine-induced immune responses did not correlate with protective efficacy.
During efficacy studies, vaccine strain shedding was confirmed by viral culture. Shedding occurred in low amounts and was detected in 5–13% of subjects 3–5 days after the first dose, but in <1% thereafter, including shedding after the second and third vaccine doses.[103–105] However, using the more sensitive molecular-based techniques, a post-licensure study found 21% of 103 vaccinated children shed PRV strains 6–8 days after the first dose.[107] In addition, a recent report found evidence for transmission of PRV-derived P[8]G1 vaccine-virus reassortant strain from a vaccinated infant to an unvaccinated sibling, resulting in symptoms of gastroenteritis and emergency department care.[108]
PRV does not interfere with other routinely administered vaccines, and although co-administered oral polio vaccines may reduce serum antirotavirus IgA geometric mean titers, rates of seroconversion remain high and comparable with seroconversion rates when oral polio vaccine administration is delayed.[109,110]
5.1.2 Efficacy
The pivotal phase III trial to determine PRV efficacy (REST; Rotavirus Efficacy and Safety Trial) was conducted in more than 70 000 infants, who were mainly from Western Europe and the US.[104] Table I summarizes the results of this and other phase III trials, including the extension of REST in European recruits and preliminary results from field trials completed in Africa and Asia recently.
Major phase III field trials of the multiple strain human-bovine pentavalent reassortant rotavirus vaccine (modified from Grimwood and Lambert,[40] with permission)
In REST, PRV reduced clinic visits, emergency department attendance, and hospital admissions for rotavirus gastroenteritis during the first 12 months of life by 86% (95% CI 74, 93), 94% (95% CI 89, 97), and 96% (95% CI 90, 98), respectively, and hospitalization for gastroenteritis from any cause by 59% (95% CI 52, 67).[104] The results were consistent across study sites in Europe, the US and Latin America.[115] In a nested substudy of 5673 infants, PRV reduced all cases of rotavirus gastroenteritis by 74% (95% CI 67, 79). Table I shows that when REST was extended for a second rotavirus season in more than 30 000 European infants, these high levels of protective efficacy were maintained.[111] In yet another variation of REST, the Finnish Extension Study,[116] almost 21 000 Finnish infants were followed for >3 years after receiving their third dose of the vaccine. Protective efficacy against severe disease resulting in presentation to the emergency department or hospital admission remained high in the Finnish cohort at 94% (95% CI 91, 96) for at least 3 years following completion of vaccination, by which time there was a marked reduction in children seeking healthcare for rotavirus gastroenteritis. It is worth noting that during REST, serotype G1 strains predominated and relatively few cases were caused by other G types. Post hoc analyses also showed PRV to be highly efficacious in 2070 infants born between 25 and 36 gestational weeks[117] and among 1566 exclusively breast fed infants.[118] Preliminary data suggest that preterm infants may shed rotavirus antigen at higher than expected rates for up to 2 weeks following their first dose, although this needs further confirmation, and its clinical significance is uncertain.[119] Incomplete vaccination with PRV appears to provide some protection against severe rotavirus disease. In REST, one and two doses of PRV reduced emergency department and hospital admissions by 82% (95% CI 39, 97) and 84% (95% CI 54, 96), respectively.[120]
With the success of REST, attention turned to the low income countries of Africa and Asia, where rotavirus vaccines have the greatest potential to reduce rotavirus mortality. The WHO deemed that efficacy studies were needed in these settings as early rotavirus vaccines proved less immunogenic in tropical countries and failed to protect African infants.[121] Interference from maternal antibodies, severe malnutrition, co-infection with other enteric pathogens, chronic illness (including HIV), and difficulties maintaining the cold chain may have contributed to the reduced efficacy of live-attenuated oral vaccines.[122]
Phase III trials of PRV in low income countries of Africa and Asia have recently been completed and their initial results have been made available (table I).[112,113] The vaccine was administered at 6, 10 and 14 weeks of age, along with other routine vaccinations; breast feeding was not restricted and infants with HIV were not excluded. The protective efficacy against severe rotavirus gastroenteritis was 51–64%, which is similar to results of a post-licensure case-control study from Nicaragua.[123]
5.1.3 Safety
Combined safety data from 71 799 infants participating in three large phase III trials, including REST, show that PRV is safe and well tolerated.[124] Importantly, there was no evidence of increased intussusception risk: within 42 days of any dose, intussusception occurred in six PRV and five placebo recipients. No clustering of cases was observed and within 1 year of receiving PRV, 13 infants experienced intussusception compared with 15 who had been given placebo (relative risk 0.9; 95% CI 0.4, 1.9). Close monitoring of 11 722 immunized subjects showed no difference in rates of fever or irritability between PRV and placebo recipients, although vomiting and diarrhea within 7 days of the first dose occurred in slightly more recipients receiving PRV (10.4% vs 9.1% and 6.7% vs 5.4%, respectively). These symptoms were predominantly of a mild nature. Similarly, PRV was well tolerated by 154 healthy preterm infants in a nested substudy of REST.[117]
After delivery of about 22 million doses, intensive post-licensure monitoring within the US employing both active and passive surveillance systems has not found an association between PRV administration and risk of intussusception, gastrointestinal bleeding, or Kawasaki syndrome.[125,126] However, recent reports of severe and protracted vaccine-related rotavirus diarrhea in infants with severe combined immunodeficiency following the first or second dose of PRV[47] has led to both licensed rotavirus vaccines being contraindicated when this disorder is present.[127] This experience in infants with severe combined immunodeficiency and transmission of a vaccine-derived reassortant rotavirus strain between siblings,[108] highlight again the need for caution when using live vaccines in infants with known severe immunodeficiency from any cause or with seriously immunocompromised household contacts.
5.2 Single Human Strain Rotavirus Vaccine (HRV)
5.2.1 Profile
Human strain rotavirus vaccine (HRV; RIX4144, Rotarix®, GlaxoSmithKline, Rixensart, Belgium) is a live oral vaccine that contains a single human rotavirus strain, G1P[8], which was first isolated from a child in 1988, before being attenuated by repeated passaging in African green monkey kidney cells and Vero cells.[100] It is stored at 2–8°C as either a lyophilized powder that requires reconstitution, or as a ‘ready to use’ liquid formulation in some countries. HRV is administered in a two-dose schedule, beginning between 6–14 weeks of age, and the second dose is completed by the age of 8 months (upper age limit varies between countries) with at least a 4-week interval between doses. HRV does not cause interference with other routinely administered vaccines.[128,129] Moreover, oral polio vaccine does not significantly impair HRV immunogenicity in infants from low and middle income regions of the world.[129,130]
As a single live attenuated human rotavirus strain, HRV seeks to imitate the protection that develops following natural infection by inducing both homotypic and heterotypic immunity. Although it differs antigenically from commonly circulating non-G1 strains, it usually shares the VP4 neutralizing antigen with G3, G4, and G9 strains. Cross protection for G2P[4] and emerging or less common strains, such as G12P[6], will depend upon shared cross-reacting VP4 epitopes and other non-VP7/VP4 proteins. Rates of IgA seroconversion following HRV vaccination are dose dependent and vary between high, middle, and low income countries. At higher vaccine doses, IgA seroconversion rates were 78–96% in infants from North America, Singapore and Finland,[131–133] 65–71% in Latin America,[134,135] and only 53–67% in infants from Africa, India and Bangladesh.[129,136,137] A possible explanation in part for the lower immunogenicity and efficacy of rotavirus vaccines in low income countries comes from a recent study, which observed higher rotavirus-specific IgA antibody titers and neutralizing activity in the breast milk of mothers from India compared with the breast milk of North American women.[138] However, seroconversion rates in HIV-infected infants were similar to those in non-HIV-infected African infants.[136] Compared with PRV, there seems to be better association between HRV IgA seroconversion and the level of protection against severe disease.[139] HRV appears to replicate more efficiently than PRV in the infant gut. Following the first dose of HRV, both antigen and live virus shedding are detected in 50–58% and 15–25% of infants, respectively.[140] Shedding of the vaccine strain can persist for 1–3 weeks. However, lower rates and shorter duration of shedding occur after the second vaccine dose. Horizontal transmission of HRV vaccine virus among twins is being studied in the Dominican Republic, and while such transmission will likely contribute to herd immunity, it might also pose risks to immunocompromised household members.[47,108]
5.2.2 Efficacy
Table II outlines the major phase III trials for HRV efficacy in various settings. The largest safety and efficacy study enrolled 63 225 infants, mainly from Latin America and Finland.[141] A nested efficacy substudy of 20 169 infants followed until 12 months of age showed that HRV reduced severe rotavirus gastroenteritis requiring oral or intravenous rehydration according to WHO guidelines and/or hospitalization by 85% (95% CI 72, 92). In an extension of this study involving 15 183 infants from Latin America, HRV maintained a protective efficacy of 79% (95% CI 66, 87) during a second rotavirus season and an overall efficacy for the first 2 years of life of 81% (95% CI 71, 87) against severe rotavirus gastroenteritis.[142]
Major phase III field trials of the single human rotavirus vaccine (modified from Grimwood and Lambert,[40] with permission)
Large phase III clinical trials have also been conducted in high income countries of Western Europe and Asia.[143,144] In these settings HRV proved highly efficacious, providing 90–96% protection against severe rotavirus gastroenteritis (Vesikari score ≥11)[148] and reducing hospitalizations for rotavirus disease by 94–96% by 2 years of age. In Europe, hospitalizations from all causes of gastroenteritis in vaccine recipients were reduced by an impressive 72% (95% CI 53, 83), suggesting that there was an even greater burden of rotavirus disease than previously realized or that rotavirus vaccines may also protect somehow against other enteric viral pathogens.[139,143] For 8687 Asian children immunized as infants, HRV continued to provide protection against severe episodes of rotavirus-related illness (100%; 95% CI 68, 100) and hospital admission (100%; 95% CI 61, 100) during the third year of life.[149]
Results have recently been published from completed HRV trials in South Africa and Malawi.[145] Enrolled infants included those with HIV, no restrictions were placed upon breast feeding, and HRV was administered in either a two- or three-dose series alongside other routinely administered vaccines at 6, 10, and 14 weeks of age. Vaccine efficacy against severe disease (Vesikari score ≥11) during the first year of life was 61% (95% CI 44, 73) and did not differ significantly between those receiving either two or three doses of the vaccine. Although the vaccine efficacy of HRV was lower in Malawian infants (49%; 95% CI 19, 68) than in infants from South Africa (77%; 95% CI 56, 88), the number of severe rotavirus episodes prevented in Malawian infants was greater (6.7 vs 4.2 per 100 vaccinees) because of the higher incidence of severe gastroenteritis in Malawian than South African children. Among HRV recipients in both countries, the incidence of severe gastroenteritis from any cause was reduced by 30% (95% CI 15, 42). As 40% of Malawian infants receiving placebo in this study were already exposed to rotaviruses before they entered the efficacy surveillance period, the protection provided by prior rotavirus infection may have led to an underestimate of vaccine efficacy.
Since HRV contains a single attenuated human rotavirus strain, G1P[8], there is much interest in its efficacy against fully G and P heterotypic strains, such as G2P[4]. While HRV has been shown to have high levels of protection against severe rotavirus gastroenteritis from G-P homotypic G1P[8] (82–100%) and P homotypic G3P[8], G4P[8], and G9P[8] (81–94%) strains in phase III trials from Latin America, Europe and Asia, only in Europe were there sufficient numbers of G2P[4] strains circulating to demonstrate a protective effect against any (58%; 95% CI 10, 81) and severe (86%; 95% CI 24, 99) G2P[4] rotavirus disease.[143] An integrated analysis of three phase II and two phase III trials, involving 23 955 infants from middle and high income regions of Latin America, Europe and Asia, reported HRV efficacy against G2P[4] was 81% (95% CI 32, 96) for any rotavirus illness and 71% (95% CI 20, 91) for severe disease.[150]
Recent results of phase III trials in African infants exposed to a diverse range of rotavirus genotypes, including G1, G2, G3, G8, G12, P[4], P[6], and P[8], are of considerable interest. In pooled results from South Africa and Malawi, vaccine efficacy during infancy against severe disease (Vesikari score ≥11) caused by G1 was 64% (95% CI 30, 82), G2 79% (95% CI 9, 97), G3 84% (95% CI 10, 98), G8 64% (95% CI 17, 85), G12 52% (95% CI <0, 78), P[4] 71% (95% CI 38, 87), P[6] 55% (95% CI <0, 81), and P[8] 59% (95% CI 33, 75).[146] Furthermore, in South African infants, HRV efficacy against severe G-P homotypic G1P[8] gastroenteritis was 70% (95% CI 33, 87) and for heterotypic G2P[4] and all P[4] strains it was 92% (95% CI 32, 100) and 95% (95% CI 60, 100), respectively.[147] Taken together, these results highlight our incomplete understanding of protective immunity, which cannot be mediated solely by external capsid neutralizing antigens when a single human rotavirus vaccine strain protects against G-P homotypic strains, P homotypic and heterotypic strains, and viruses that are fully heterotypic for both G-P types and the NSP4 genogroup (G2P[4], DS-1).
Breast feeding in infants from high and middle income countries, preterm delivery and mild malnutrition do not alter immunogenicity or vaccine efficacy.[141,151,152] Although numbers were small, the European phase III trial found HRV provided 90% (95% CI 9, 100) protection between the first and second doses, suggesting HRV provides early protection when administered during the annual rotavirus epidemics.[143]
5.2.3 Safety
Integrated results of safety data from four phase II and four phase III randomized, double-blind, placebo-controlled studies conducted in the Americas, Europe and Asia and involving 71 209 infants aged 6–18 weeks, found HRV was safe and well tolerated.[153] No increased risk for intussusception was observed. Within 31 days of any dose there were nine infants with intussusception in the vaccine group and seven in the placebo group (relative risk [RR] 1.23; 95% CI 0.4, 3.9). Indeed, in a subset of 20 169 infants followed up to 12 months of age there was a suggestion that HRV could protect against intussusception later in infancy, as four cases of intussusception occurred in the vaccine group and 14 in placebo recipients (RR 0.28; 95% CI 0.10, 0.81).[139] With the exception of mild coryzal symptoms, there was no imbalance in adverse events between HRV and placebo groups, indicating the vaccine had a good reactogenicity and safety profile.
On March 22, 2010, the US FDA recommended a temporary suspension of HRV after porcine circovirus (PCV)-1 DNA fragments were detected in the vaccine, including the cell bank and viral seed. On May 7, 2010, the FDA reported DNA from PCV-1 and PCV-2 had also been detected in PRV batches. PCV is a small circular DNA virus commonly found in certain meat and food products, and it is not known to be pathogenic in humans. On May 14, 2010, the FDA updated its recommendations,[154] specifically requesting that healthcare professionals continue to use PRV and to resume HRV vaccination. This decision was made following a review of clinical trial data from >100 000 subjects and post-licensure experience involving almost 100 million infants. Also taken into consideration was the lack of evidence that PCV poses a safety risk to humans and the known substantial benefits of rotavirus vaccines. The WHO, European Medicines Agency, and the Australian Therapeutic Goods Administration also recommend continued use of rotavirus vaccines while the manufacturers correct their processes and ensure both vaccines are free of PCV DNA.[155–157]
6. Vaccine Effectiveness
Since publication of the two pivotal rotavirus vaccine trials in 2006 (see sections 5.1.2 and 5.2.2), PRV has been licensed in more than 90 countries and included in at least seven national vaccination schedules, while HRV is licensed in more than 125 countries and incorporated into the universal vaccination programs of 24 countries. Although effectiveness data are limited and mostly of an ecological nature, they suggest rotavirus vaccines have indirect as well as direct effects on rotavirus activity, and that the performance of both vaccines parallels the results of efficacy studies conducted in different settings where protection is inversely related to ‘all-cause’ mortality in children <5 years of age (table III).[113]
Rotavirus vaccine efficacy for severe gastroenteritis in the first year of life by mortality quartile (modified from the WHO,[113] with permission)
6.1 PRV
PRV was licensed in the US in 2006 and rotavirus activity declined shortly thereafter. Data from a national laboratory surveillance network showed that compared with 2000–6, the subsequent rotavirus seasons were significantly delayed, shorter in duration, and of lower magnitude.[158,159] The seasonal onset was delayed by 6–11 weeks and its duration by 9–12 weeks. The numbers of laboratory-confirmed rotavirus cases in 2007–8 and 2008–9 were 59–64% lower than annual median 2000–6 values. Similarly, the proportion of rotavirus tests that were positive decreased 60–64% from an annual median of 25% of total tests between 2000–6 to 9% in 2007–8 and 10% in the 2008–9 seasons.[159] This decline in rotavirus activity occurred in a setting where, by December 2007, 58% of eligible children had received at least one dose of PRV and just 31% had completed the full three-dose series.[158]
A nationwide health insurance claims-based post-licensure study in the US compared concurrent cohorts of children who received three doses of PRV with those who received three doses of DTaP (diphtheria, tetanus, pertussis) but no doses of PRV.[160] By comparing these two cohorts of infants, vaccine effectiveness of PRV at preventing rotavirus and acute gastroenteritis coded hospitalization and emergency department attendance during the rotavirus season was estimated at 100% (95% CI 87, 100) and 59% (95% CI 47, 68), respectively. Another multi-state hospital discharge database study covering almost 50% of the US population estimated that PRV decreased hospitalizations for acute gastroenteritis in children <5 years of age in 2008 by 45%, including those who were either too young or too old to receive the vaccine.[161] In other words, PRV in the US reduced acute gastroenteritis hospital admissions in children <5 years of age in 2008 by an estimated 55 000 cases thereby preventing about 1 in every 20 hospitalizations in that year for this age group. These national data are supported by a recently published case-control study from Houston, Texas, which reaffirmed the REST findings of partial immunization providing substantial protection.[120,162] In this particular setting, one and two doses of PRV reduced hospitalization and emergency department visits for rotavirus gastroenteritis by 69% (95% CI 13, 89) and 81% (95% CI 13, 96), respectively, compared with 88% (95% CI 68, 96) protection conferred by the full three-dose series. Meanwhile, hospitals in the US are also reporting fewer healthcare-associated rotavirus infections.[163]
Similar findings were observed using routinely collected notification and laboratory results datasets in Queensland, Australia, where PRV was introduced into the vaccination schedule in July 2007.[164] During 2007 and 2008, rotavirus cases in the vaccine-eligible target age group of those aged <2 years declined by 53% and 68%, respectively, compared with 2006 pre-vaccine notifications. Furthermore, rotavirus notifications during this period also declined by 56–65% in those aged 2–4 years who would not have received the vaccine. Figure 3 further illustrates the changes in rotavirus notifications since the introduction of PRV into Queensland.
The higher than expected performance of rotavirus vaccines in both the US and Australia[158–160,164] may result from an underestimated true burden of disease as well as indirect vaccine effects where immunized infants decrease transmission of rotaviruses to older and unimmunized children. However, longer surveillance periods are required to adjust for secular trends in rotavirus activity, including changing genotypes and other factors, which may influence vaccine performance.[165]
A case-control study conducted 1 year after PRV was introduced into the national vaccination schedule of Nicaragua provides important information on vaccine performance in low income countries with a high diarrheal disease burden.[123] Although 77% of the birth cohort had received three doses of PRV, its effectiveness at preventing hospitalization or the need for intravenous rehydration for rotavirus gastroenteritis was just 46% (95% CI 18, 64). As in other Latin American countries at the time of the study, G2P[4] was the predominant circulating rotavirus strain.[166] Efficacy data for PRV against G2P[4] are limited and based upon small case numbers (table I).[104,111] It is possible the vaccine may not be as active against this strain as it is against strains with the P[8] genotype. Nonetheless, as with protection following a wild-type rotavirus infection, vaccine performance improved with increasing disease severity, being 58% (95% CI 30, 74) for severe (Vesikari score ≥11) and 77% (95% CI 39, 92) for very severe (Vesikari score ≥15) rotavirus diarrhea. These results are similar to those from PRV efficacy studies in low income countries of Africa and Asia,[112,113] indicating the differences in vaccine effectiveness between high and low income countries are likely to be site specific.
6.2 HRV
After the introduction of HRV into the national vaccination schedule of Brazil in 2006, initial reports observed significant reductions in the proportion of gastroenteritis cases due to rotaviruses, and the almost complete replacement of other circulating rotavirus strains by fully heterotypic G2P[4] strains.[166–168] This raised concerns over the differential protective effects of HRV against heterotypic strains (see section 5.2.2), which may have been exaggerated by coincident re-emergence of G2P[4] strains throughout Latin America and in other parts of the world where rotavirus vaccines were not widely used. Reassuringly, and despite G2P[4] strains being responsible for almost 80% of all hospitalized rotavirus cases in El Salvador in 2006 when HRV was introduced, a year later homotypic G1P[8] strains predominated, accounting for about 90% of rotavirus hospital presentations, while G2P[4] accounted for only 2% of strains detected in these patients.[168] Recently, another ecological study reported that diarrheal admissions in North East Brazil in 2008 were >50% lower than 2003 hospital admission data, and the proportion of cases due to rotaviruses fell from 24% in 2006 to 10% and 7% in 2007 and 2008, respectively.[169] Vaccine coverage in this region was between 80% and 90% and, as all but one case was from a G2P[4] bearing strain, the screening method estimated that HRV reduced the risk of hospital presentation for gastroenteritis from G2P[4] strains by 89% (95% CI 87, 92). However, on a cautionary note, waning immunity against G2P[4] may be a problem. In another study from North East Brazil — in this instance a case-control study involving 926 children hospitalized for acute diarrhea who were age-eligible to receive HRV — vaccine effectiveness against G2P[4] strains in children aged 6–11 months causing severe disease and hospitalization was 77% (95% CI 42, 91) and 85% (95% CI 54, 95), respectively.[170] In contrast, the vaccine failed to protect older children. This finding was unexpected, as HRV efficacy against G-P and P-homotypic strains was sustained previously in Latin American children until at least 2 years of age,[142] and also for the same period against G2P[4] strains in European infants.[143] Meanwhile, a case-control study from El Salvador in 2007–9 found that HRV reduced hospitalization for rotavirus diarrhea from G1P[8] strains by 76% (95% CI 64, 94).[171] However, as in Brazil, protection was significantly lower in children older than 12 months of age (59%, 95% CI 27, 77) than in those aged between 6 and 11 months (83%, 95% CI 68, 91). Nevertheless, in both studies the numbers of cases in older children were relatively small. Second year follow-up data from clinical trials in Africa and ongoing surveillance will help determine whether rotavirus vaccines maintain their effectiveness in children from low and middle income countries.
Pre-licensure trials of rotavirus vaccines were conducted in populations with relatively low mortality rates from diarrhea. However, of considerable importance, a recent post-licensure study from Mexico found that in 2008, the year following introduction of HRV into the national immunization program, there was a 41% (95% CI 36, 47) reduction in all-cause diarrhea-related mortality among infants eligible for the vaccine.[172] Diarrhea-related deaths were also 29% (95% CI 17, 39) lower in 1- to 2-year-old children, few of whom would have received the vaccine. The decreased fatality rates were sustained the following year, and the decreases in mortality in both years were most evident during winter, where diarrhea-related deaths in children <2 years of age were two-thirds of pre-vaccine levels. Even though this was an ecological study, the data suggest HRV helped to reduce diarrheal deaths in Mexican children and also provided additional protection by reducing exposure to rotavirus in older unvaccinated children.
Another means of determining vaccine effectiveness is during an outbreak. A few months after introducing HRV into central Australia, a widespread rotavirus outbreak occurred in 2007 involving remote rural Aboriginal communities where diarrheal disease burden is especially high.[73,74] This outbreak was caused by G9P[8] strains, and a subsequent case-control study reported vaccines reduced the risk of hospitalization for gastroenteritis and proven rotavirus disease by 78% (95% CI 40, 92) and 85% (95% CI 23, 97), respectively.[173] In 2009 there was another rotavirus outbreak in central Australia. On this occasion it was caused by G2P[4]. Similar to the experience in Latin America,[170,171] HRV was highly effective at preventing severe G2P[4] rotavirus disease in Australian Aboriginal infants younger than 12 months of age, but failed to protect older vaccinated children.[174] Overall, these post-licensure studies provide further reassurance of HRV performance during infancy, although questions remain over its capacity to provide sustained protection in high burden, impoverished settings.
7. Potential Economic Benefits of Rotavirus Vaccines
7.1 Costs of Rotavirus Gastroenteritis
Rotavirus gastroenteritis in infants and children is associated with direct medical and non-medical costs, as well as indirect costs. The main medical costs are hospital admissions and ambulatory presentations to the hospital or family physician for diagnostic tests, medication, oral rehydration therapy, and supplementary therapies.[175] In addition, there are considerable medical and non-medical costs to the family, including co-payments on oral rehydration therapy, sanitary supplies such as additional diapers (nappies) for infants and young children, transportation to medical care, plus indirect costs of loss of income by caregivers, and potential lost future income from premature deaths of children.
In high income countries, medical costs of rotavirus gastroenteritis are moderately high and mortality is relatively low. A large study (REVEAL; Rotavirus gastroenteritis Epidemiology and Viral types in Europe Accounting for Losses in public health and society) of rotavirus gastroenteritis in young children was conducted in selected areas of Belgium, France, Germany, Italy, Spain, Sweden, and the UK in 2004–5.[175] The total societal cost, including direct medical, direct non-medical and indirect costs per episode of rotavirus gastroenteritis, ranged from €166 to €473 in the primary care setting, from €334 to €770 in the emergency department and from €1525 to €2101 in the hospital setting in 2006 values. The mean number of workdays lost by parents and other relatives was 2.3–7.5 days per episode.
Treatment costs for rotavirus diarrhea are less in low and middle income countries, but mortality rates are much higher. A recent study concluded that rotavirus accounts for 27 million medical visits and 527 000 deaths annually worldwide; 45% of these deaths were in Africa and 30% in Asia. The annual cost across all developing countries in the absence of any rotavirus vaccination program was estimated at $US423 million or $US3.47 per child.[22]
7.2 Cost Effectiveness of Rotavirus Vaccination Programs
Economic evaluations of vaccination programs synthesize clinical, epidemiological and economic information in the form of economic models. Although broad principles for economic modeling of pharmaceuticals, including vaccines, are well established,[176] the results of economic models are specific to the country or region under consideration.
The cost effectiveness of any vaccination program depends largely on the efficacy and cost of the vaccine, the incidence and prevalence of the condition under consideration, and the mortality and morbidity associated with the disease. In most settings, both of the currently available rotavirus vaccines are highly efficacious against severe rotavirus diarrhea (see sections 5.1.2 and 5.2.2), and this finding is generalizable across most countries. Arguably, the transient reduction in a child’s quality of life associated with an episode of rotavirus diarrhea is also generalizable, although this parameter is somewhat uncertain. The remaining determinants of cost effectiveness are specific to the local environment: the cost per vaccine course including administration and wastage; severe rotavirus disease incidence rates; the cost of treatment; and (importantly) the case fatality ratio, which is largely a measure of access to medical care. The outputs of the economic model can include the incremental cost per hospital admission averted and/or per case or death averted, and/or the cost per life-year gained or quality-adjusted life-year (QALY) gained or disability-adjusted life-year (DALY) averted by a vaccination program.
Coverage by a rotavirus vaccination program clearly affects the budget impact as well as the net health benefit to the country concerned. If the indirect benefits from herd immunity suggested by several post-licensure ecological studies are confirmed,[158,159,164,169] rotavirus vaccination programs will become more effective and, therefore, more cost effective as the coverage increases beyond some critical level. In low and middle income countries with relatively poor access to healthcare, rotavirus immunization programs would prevent childhood fatalities and thereby gain life years. In this situation the discount rate for future life-years gained would also become an important determinant of cost effectiveness.
There is no commonly agreed universal threshold for an acceptable cost per QALY gained (or DALY averted) for vaccination programs, mainly because the affordability of any new technology depends on the local availability of healthcare funding and the availability of other new technologies competing for the same budget. The WHO has defined ‘cost-effective’ interventions as those that avert each additional DALY at a cost less than 3 times the gross domestic product (GDP) per capita.[177] By this criterion, the cost effectiveness of rotavirus vaccination programs in each country depends largely on the case fatality (which determines DALYs averted) and the price of the vaccine in relation to the per capita GDP. A case can be made for funding of vaccines to be considered differently from other pharmaceuticals because of their preventative nature and public health benefits, including a long duration of benefit and potential for herd effects.[178]
Economic evaluations of rotavirus vaccination programs in high, middle and low income countries have been reviewed recently.[179] Most of these studies utilized static deterministic models, which exclude herd effects and therefore could underestimate the economic impact of rotavirus vaccination programs with high coverage. There is a broad range of results across various countries, reflecting largely the local vaccine price and severe rotavirus disease incidence and mortality rates, which vary widely. Most measurable costs of rotavirus illness in high income countries comprise direct medical costs, with lost parental income making up as much as one-third of the cost to society.[175] Some studies take into account the impact of rotavirus diarrhea on the quality of life of one or two caregivers,[180–183] and others include lifetime productivity losses attributable to infant deaths.[18] In the US, where at least 50% of infants have received at least one vaccine dose, rotavirus vaccines are predicted to prevent 313 000 hospitalizations for rotavirus gastroenteritis among children <5 years of age over the period 2006–15.[184] In New Zealand, which has an annual birth cohort of approximately 65 000 and where childhood gastroenteritis is common but seldom fatal, about six children are required to be vaccinated to avoid one primary care consultation, 49 to avert one hospitalization, and 73 000 to prevent one death. The cost per QALY is about $US27 000, in year 5 of the program (in 2006/7 dollars), which is cost effective by international standards.[183] Herd protection is likely to improve the cost effectiveness greatly. Inclusion of cases that do not seek medical care, and the expected indirect benefits of reduced episodes of healthcare-associated rotavirus gastroenteritis and outbreaks in nursing homes, could further strengthen the case for government funding of rotavirus vaccination programs, although it could also reduce the credibility of the analyses because of the uncertain prevalence and costs associated with all of these cases.
A recent study estimated the cost effectiveness of rotavirus vaccination programs in low and middle income countries in Africa, the Americas, the eastern Mediterranean, Europe, Southeast Asia, and the western Pacific.[22] Rotavirus vaccination would prevent 228 000 deaths, 13.7 million hospital visits and 8.7 million DALYs annually, saving in 2007 dollar values $US188 million in treatment costs and $US243 million in societal costs. More broadly, in 64 low income countries, introduction of rotavirus vaccination programs over a period of 18 years is predicted to prevent 2.4 million deaths and over 80 million DALYs, with cost effectiveness improving over time as the unit price of the vaccine declines. Thus, the potential introduction of rotavirus vaccines into the world’s poorest countries is very cost effective and is projected to reduce child mortality substantially.[185] However, the cost effectiveness of such a strategy depends upon the vaccine prices that can be negotiated across these countries.
Mortality can also have intangible effects on funding decisions. When vaccines against different diseases are competing for limited public funding, the ‘rule of rescue’ can prevail. Vaccination programs that prevent childhood deaths are more likely to attract funding than vaccines that prevent common non-fatal illnesses, regardless of the findings of economic evaluations.[186] As a result, in high income countries with low mortality from childhood gastroenteritis, rotavirus vaccines are less likely to attract funding than other new vaccines that prevent deaths, such as pneumococcal and human papillomavirus vaccines.[186] However, in developing countries with high mortality from rotavirus gastroenteritis, vaccination programs are likely to achieve a more favorable status, provided the vaccine is priced appropriately. Ultimately, whether rotavirus vaccination programs are likely to attract government or state funding depends largely on the local price of the vaccine, the local mortality rate from rotavirus diarrhea, the per capita GDP, and the available budget. The main economic challenges are negotiating lower vaccine prices for developing countries, and educating governments and healthcare providers of these countries about the economic and health benefits of rotavirus vaccine programs.
8. The Future
In 2009, the WHO made a recommendation for infants worldwide to be immunized against rotaviruses.[113,187] This recommendation followed the completion of studies in low income countries of Africa and Asia (tables I–III), which showed PRV and HRV were relatively immunogenic and efficacious in these settings, and the demonstration of post-licensure vaccine safety and effectiveness in high, middle and low income countries of the Americas (see sections 5 and 6).
8.1 Research Questions
Despite the success of currently licensed rotavirus vaccines, several important questions remain. A greater understanding of natural and vaccine-induced protective immunity will help with the development of the next generation of vaccines to improve immunogenicity in the neonatal period and in settings where there is a high diarrheal burden, diverse rotavirus genotypes, and poor environmental hygiene.[73,188]
Confirmation of the indirect protective effects of rotavirus vaccines to unimmunized infants and older age groups is needed to ensure that cost-effectiveness models are optimized and public health policy makers are made aware of these potential benefits. This is especially true for low income countries. As already seen with pneumococcal conjugate vaccines,[189] herd protection is likely to result from reduced rotavirus transmission in the community. However, direct transmission of vaccine strains between immunized infants and unimmunized contacts may also contribute. Ongoing transmission studies in twins will provide some answers and help determine the potential risk of vaccine strains to immunocompromised household contacts.
Recent studies show that 30% of Bangladeshi and 40% of Malawian infant subjects have IgA serological evidence of prior rotavirus infection at age 8 and 18 weeks, respectively.[136,145,190] Moreover, approximately 30% of infants from low and middle income countries do not receive their first vaccinations until after 12 weeks of age.[191] Countries with the greatest mortality rates from rotavirus gastroenteritis are more likely to have delayed vaccine coverage. For example, in Nigeria, where 50 000–70 000 children are estimated to die annually from rotavirus infections,[5,12,192] <25% of infants receive their initial routine vaccinations before the age of 3 months.[193] Taken together, the early onset of primary infection and poor access to medical care and vaccination services pose the greatest challenges facing rotavirus programs in low income countries. A possible solution is to hasten the development of vaccines that can be given to newborn babies, and to collect safety and efficacy data urgently to allow a loosening of the age restrictions on rotavirus vaccine administration.[194,195] A criticism of vaccines administered in the newborn period is their poor immunogenicity resulting from blocking maternal antibodies and the baby’s immature immune system. However, unique wild-type neonatal rotavirus strains RV3 (G3P[6]) and 116E (G9P[11]) have already been shown to replicate well in the presence of maternal antibodies and to induce high level protective heterotypic immunity.[7,88] Candidate oral rotavirus vaccines derived from these neonatal strains are about to undergo field trials to determine whether they are safe and efficacious in young infants and newborn babies.[7]
Finally, before introducing rotavirus vaccine programs, it is important to have surveillance systems established to identify the baseline rotavirus disease burden, temporal trends, and strain diversity. The surveillance systems can then be used to measure the impact of rotavirus vaccines on disease prevalence. These systems will also help to determine whether vaccines have strain-specific differences in their activity, especially against G2P[4] and G12P[6] in settings of poverty and high diarrhea burden. Surveillance is required for several years to monitor the effects of vaccine-induced immune selection pressure on rotavirus evolution, as novel strains might emerge, including vaccine-derived reassortant viruses, and they could compromise program effectiveness.[120,196] Such information will not only help guide public health policy, but provide valuable information on rotavirus biology, evolution, and immunity. The recent WHO global recommendation in favor of affordable rotavirus vaccines will assist with meeting the Millennium Development Goal of reducing the mortality rate in children <5 years of age by two-thirds between 1990 and 2015. If fully implemented, rotavirus vaccines could decrease the annual mortality in children <5 years of age by as much as 4%, and decrease mortality and hospital admissions for diarrhea by between 20% and 40% in this age group.[197] However, it is important to remember that rotavirus vaccines are but one component of a comprehensive approach to the prevention and management of childhood diarrheal disease.[198]
References
Parashar UD, Gibson CJ, Bresee JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12(2): 304–6
Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9(5): 565–72
Bilcke J, Van Damme P, Van Ranst M, et al. Estimating the incidence of symptomatic rotavirus infections: a systematic review and meta-analysis. PLoS ONE 2009; 4(6): e6060
Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375(9730): 1969–87
Parashar UD, Burton A, Lanata C, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200Suppl. 1: S9–15
Widdowson M-A, Steele D, Vojdani J, et al. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis 2009; 200Suppl. 1: S1–8
Danchin MH, Bines JE. Defeating rotavirus? The global recommendation for rotavirus vaccination. N Engl J Med 2009; 361(20): 1919–21
Nokes DJ, Abwao J, Pamba A, et al. Incidence and clinical characteristics of group A rotavirus infections among children admitted to hospital in Kilifi, Kenya. PLoS Med 2008; 5(7): e153
Ramani S, Kang G. Burden of disease and molecular epidemiology of group A rotavirus infections in India. Indian J Med Res 2007; 125(5): 619–32
Bahl R, Ray P, Subodh S, et al. Incidence of severe rotavirus diarrhea in New Delhi, India, and G and P types of the infecting rotavirus strains. J Infect Dis 2005; 192Suppl. 1: S114–9
Nelson EAS, Bresee JS, Parashar UD, et al. Rotavirus epidemiology: the Asian Rotavirus Surveillance Network. Vaccine 2008; 26(26): 3192–6
Sanchez-Padilla E, Grais RF, Guerin PJ, et al. Burden of disease and circulating serotypes of rotavirus infection in sub-Saharan Africa: systematic review and meta-analysis. Lancet Infect Dis 2009; 9(9): 567–76
de Oliveira LH, Danovaro-Holliday MC, Matus CR, et al. Rotavirus vaccine introduction in the Americas: progress and lessons learned. Expert Rev Vaccines 2008; 7(3): 345–53
de Oliveira LH, Danovaro-Holliday MC, Andrus JK, et al. Sentinel hospital surveillance for rotavirus in Latin American and Caribbean countries. J Infect Dis 2009; 200Suppl. 1: S131–9
Lepage P. Rotavirus infection in Europe: time for effective prevention? Pediatr Infect Dis J 2006; 25(1): S5–6
Flores AR, Szilagyi PG, Auinger P, et al. Estimated burden of rotavirus-associated diarrhea in ambulatory settings in the United States. Pediatrics 2010; 125(2): e191–8
Fischer TK, Viboud C, Parashar U, et al. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003. J Infect Dis 2007; 195(8): 1117–25
Widdowson M-A, Meltzer MI, Zhang X, et al. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics 2007; 119(4): 684–95
Mast TC, Walter EB, Bulotsky M, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J 2010; 29(2): e19–25
Soriano-Gabarro M, Mrukowicz J, Vesikari T, et al. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis 2006; 25(1): S7–11
Standaert B, Harlin O, Desselberger U. The financial burden of rotavirus disease in four countries of the European Union. Pediatr Infect Dis J 2008; 27(1): S20–7
Rheingans RD, Antil L, Dreibelbis R, et al. Economic costs of rotavirus gastroenteritis and cost-effectiveness of vaccination in developing countries. J Infect Dis 2009; 200Suppl. 1: S16–27
Galati JC, Harsley S, Richmond P, et al. The burden of rotavirus-related illness among young children on the Australian health care system. Aust NZ Public Health 2006; 30(5): 416–21
Harris JP, Jit M, Cooper M, et al. Evaluating rotavirus vaccination in England and Wales: Part I, estimating the burden of disease. Vaccine 2007; 25(20): 3962–70
Wheeler JG, Sethi D, Cowden JM, et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reporting to national surveillance. BMJ 1999; 318(7190): 1046–50
Cortes JE, Curns AT, Tate JE, et al. Trends in healthcare utilization for diarrhea and rotavirus disease in privately insured US children <5 years of age, 2001–2006. Pediatr Infect Dis J 2009; 28(10): 874–8
Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe gastroenteritis in children in the United States. Pediatrics 2008; 122(6): 1235–43
Van Damme P, Giaquinto C, Huet F, et al. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis 2007; 195Suppl. 1: S4–16
Forster J, Guarino A, Parez N, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123(3): e393–400
Grimwood K, Huang QS, Cohet C, et al. Rotavirus hospitalisation in New Zealand children under 3 years of age. J Paediatr Child Health 2006; 42(4): 196–203
Gutierrez-Gimeno MV, Martin-Moreno JM, Diez-Domingo J, et al. Nosocomial rotavirus gastroenteritis in Spain: a multicenter prospective study. Pediatr Infect Dis J 2010; 29(1): 23–7
Cunliffe NA, Booth JA, Elliot C, et al. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis 2010; 16(1): 55–62
Gleizes O, Desselberger U, Tatochenko V, et al. Nosocomial rotavirus infection in European countries: a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis 2006; 25(1): S12–21
Fruhwirth M, Heininger U, Ehlken B, et al. International variation in disease burden of rotavirus gastroenteritis in children with community- and nosocomially acquired infection. Pediatr Infect Dis J 2001; 20(8): 784–91
Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine 2004; 22Suppl. 1: S49–54
Smith MJ, Clark HF, Lawley D, et al. The clinical and molecular epidemiology of community- and healthcare-acquired rotavirus gastroenteritis. Pediatr Infect Dis J 2008; 27(1): 54–8
Richardson SC, Grimwood K, Gorrell R, et al. Extended excretion of rotavirus after severe diarrhoea in young children. Lancet 1998; 351(9119): 1844–8
Barnes GL, Callaghan SL, Kirkwood CD, et al. Excretion of serotype G1 rotavirus strains by asymptomatic staff: a possible source of nosocomial infection. J Pediatr 2003; 142(6): 722–5
Waisbourd-Zinman O, Ben-Ziony S, Solter E, et al. Hospitalizations for nosocomial rotavirus gastroenteritis in a tertiary pediatric center: a 4-year prospective study. Am J Infect Control 2009; 37(6): 465–9
Grimwood K, Lambert SB. Rotavirus vaccines: opportunities and challenges. Hum Vaccines 2009; 5(2): 57–69
Staat MA, Azimi PH, Berke T, et al. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J 2002; 21(3): 221–7
Grimwood K, Carzino R, Barnes GL, et al. Patients with enteric adenovirus gastroenteritis admitted to an Australian pediatric teaching hospital from 1981–1992. J Clin Microbiol 1995; 33(1): 131–6
O’Ryan ML, Lucero Y, Prado V, et al. Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr Infect Dis J 2009; 28(10): 879–84
Anderson EJ, Weber SG. Rotavirus infections in adults. Lancet Infect Dis 2004; 4(2): 91–9
Sharma R, Hudak ML, Premachandra BR, et al. Clinical manifestations of rotavirus infection in the neonatal intensive care unit. Pediatr Infect Dis J 2002; 21(12): 1099–105
Estes MK, Kapikian AZ. Rotaviruses. In: Knipe DM, Howley PM, editors. Fields virology. 5th ed. Philadelphia (PA): Lippincott, Williams and Wilkins, 2007: 1917–94
Patel NC, Hertel PM, Estes MK, et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med 2010; 262(4): 314–9
Cunliffe NA, Gondwe JS, Kirkwood CD, et al. Effect of concomitant HIV infection on presentation and outcome of rotavirus gastroenteritis in Malawian children. Lancet 2001; 358(9281): 550–5
Grimwood K, Coakley JC, Hudson IL, et al. Serum aspartate aminotransferase levels after rotavirus gastroenteritis. J Pediatr 1988; 112(4): 597–600
Kirton A, Busche K, Ross C, et al. Acute necrotizing encephalopathy in Caucasian children: two cases and review of the literature. J Child Neurol 2005; 20(6): 527–32
Ramig RF. Systemic rotavirus infection. Expert Rev Anti Infect Ther 2007; 5(4): 591–612
Blutt SE, Kirkwood CD, Parreno V, et al. Rotavirus antigenaemia and viraemia: a common event? Lancet 2003; 362(9394): 1445–9
Sugata K, Taniguchi K, Yiu A, et al. Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics 2008; 122(2): 392–7
Ward RL, Bernstein DI, Young EC, et al. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J Infect Dis 1986; 154(5): 871–80
Zahn M, Marshall GS. Clinical and epidemiological aspects of rotavirus infection. Pediatr Ann 2006; 35(1): 23–8
Ansari SA, Springthorpe VS, Sattar SA. Survival and vehicular spread of human rotaviruses: possible relation to seasonality of outbreaks. Rev Infect Dis 1991; 13(3): 448–61
Grimwood K, Abbott GD, Fergusson DM, et al. Spread of rotavirus within families: a community based study. BMJ 1983; 287(6392): 575–7
Gladstone BP, Iturriza-Gomara M, Ramani S, et al. Polymerase chain reaction in the detection of an ‘outbreak’ of asymptomatic viral infections in a community birth cohort in south India. Epidemiol Infect 2008; 136(3): 399–405
Ramani S, Arumugam R, Gopalarathanim N, et al. Investigation of the environment and of mothers in transmission of rotavirus infections in the neonatal nursery. J Med Virol 2008; 80(6): 1099–105
Zerr DM, Allpress AL, Heath J, et al. Decreasing hospital-associated rotavirus infection: a multidisciplinary hand hygiene campaign in a children’s hospital. Pediatr Infect Dis J 2005; 24(5): 397–403
Bishop RF, Masendycz PJ, Bugg HC, et al. Epidemiological patterns of rotaviruses causing severe gastroenteritis in young children throughout Australia from 1993 to 1996. J Clin Microbiol 2001; 39(3): 1085–91
Levy K, Hubbard AE, Elsenberg JNS. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol 2009; 38(6): 1487–96
Barnes GL, Uren E, Stevens KB, et al. Etiology of acute gastroenteritis in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol 1998; 36(1): 133–8
Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med 1996; 335(14): 1022–8
D’Souza RM, Hall G, Becker NG. Climatic factors associated with hospitalizations for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect 2008; 136(1): 56–64
Perez Schael I, Gonzalez R, Salinas B. Severity and age of rotavirus diarrhea, but not socioeconomic conditions, are associated with rotavirus seasonality in Venezuela. J Med Virol 2009; 81(3): 562–7
Atchison CJ, Tam CC, Lopman BA. Season of birth and risk of rotavirus diarrhea in children aged <5 years. Epidemiol Infect 2009; 137(7): 957–60
Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 2009; 325(5938): 290–4
Sethi D, Cumberland P, Hudson MJ, et al. A study of infectious intestinal disease in England: risk factors associated with group A rotavirus in children. Epidemiol Infect 2001; 126(1): 63–70
Dennehy PH, Cortese MM, Begue RE, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in US children. Pediatr Infect Dis J 2006; 25(12): 1123–31
Huppertz H-I, Salman N, Giaquinto C. Risk factors for severe gastroenteritis. Pediatr Infect Dis J 2008; 27Suppl. 1: S11–9
Grimprel E, Rodrigo C, Desselberger U. Rotavirus disease: impact of coinfections. Pediatr Infect Dis J 2008; 27Suppl. 1: S3–10
Grimwood K, Forbes DA. Acute and persistent diarrhea. Pediatr Clin Nth Amer 2009; 56(6): 1343–61
Schultz R. Rotavirus gastroenteritis in the Northern Territory, 1995–2004. Med J Aust 2006; 185(7): 354–6
Campbell SJ, Nissen MD, Lambert SB. Rotavirus epidemiology in Queensland during the pre-vaccine era. Com Dis Intel 2009; 33(2): 204–8
Ruuska T. Occurrence of acute diarrhea in atopic and nonatopic infants: the role of prolonged breast-feeding. J Pediatr Gastroenterol Nutr 1992; 14(1): 27–33
Cunliffe NA, Bresee JS, Gentsch JR, et al. The expanding diversity of rotaviruses. Lancet 2002; 359(9307): 640–1
Hoshino Y, Kapakian AZ. Rotavirus serotypes: classification and importance in epidemiology, immunity and vaccine development. J Health Popul Nutr 2000; 18(1): 5–14
Sharma S, Paul VK, Bhan MK, et al. Genomic characterization of nontypeable rotaviruses and detection of a rare G8 strain in Delhi, India. J Clin Microbiol 2009; 47(12): 3998–4005
Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implications for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 2005; 15(1): 29–56
Centers for Disease Control and Prevention (CDC). Rotavirus surveillance: worldwide, 2001–2008. Morb Mortal Wkly Rep 2008; 57(46): 1255–7
Iturriza-Gomara M, Dallman T, Banyai K, et al. Rotavirus surveillance in Europe, 2005–2008: web-enabled reporting and real-time analysis of genotyping and epidemiological data. J Infect Dis 2009; 200Suppl. 1: S215–21
Kirkwood CD, Boniface K, Bogdanovic-Sakran N, et al. Rotavirus strain surveillance: an Australian perspective of strains causing disease in hospitalised children from 1997–2007. Vaccine 2009; 27Suppl. 5: F102–7
Hasing ME, Trueba G, Baquero MI, et al. Rapid changes in rotaviral genotypes in Ecuador. J Med Virol 2009; 81(12): 2109–13
Nelson EAS, Widdowson M-A, Kilgore PE, et al. A decade of the Asian Rotavirus Surveillance Network: achievements and future directions. Vaccine 2009; 27Suppl. 5: F1–3
Cunliffe NA, Ngwira BM, Dove W, et al. Serotype G12 rotaviruses, Lilongwe, Malawi. Emerg Infect Dis 2009; 15(1): 87–90
Freeman MM, Kerin T, Hull J, et al. Phylogenetic analysis of novel G12 rotaviruses in the United States: a molecular search for the origin of a new strain. J Med Virol 2009; 81(4): 736–46
Bishop RF, Barnes GL, Cipriani E, et al. Clinical immunity after neonatal rotavirus infection: a prospective longitudinal study in young children. N Engl J Med 1983; 309(2): 72–6
Velazquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 2000; 182(6): 1602–9
Chiba S, Yokoyahama T, Nakata S, et al. Protective effect of naturally acquired homotypic and heterotypicrotavirus antibodies. Lancet 1986; 328(8504): 417–21
Coulson BS, Grimwood K, Hudson IL, et al. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol 1992; 30(7): 1678–84
Richardson SC, Grimwood K, Bishop RF. Analysis of homotypic and heterotypic serum immune responses to rotavirus proteins following primary rotavirus infection by using the radioimmunoprecipitation technique. J Clin Microbiol 1993; 31(2): 377–85
Gorrell RJ, Bishop RF. Homotypic and heterotypic serum neutralizing antibody response to rotavirus proteins following natural primary infection and reinfection in children. J Med Virol 1999; 57(2): 204–11
Yuan L, Honma S, Kim I, et al. Resistance to rotavirus infection in adult volunteers challenged with a virulent G1P1A[8] virus correlated with serum immunoglobulin G antibodies to homotypic viral proteins 7 and 4. J Infect Dis 2009; 200(9): 1443–51
Ward R. Mechanisms of protection against rotavirus infection and disease. Pediatr Infect Dis J 2009; 28Suppl. 3: S57–9
Tate JE, Curns AT, Cortese MM, et al. Burden of acute gastroenteritis hospitalizations and Emergency Department visits in US children that is potentially preventable by rotavirus vaccination: a probe study using the now-withdrawn RotaShield vaccine. Pediatrics 2009; 123(3): 744–9
Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given oral rotavirus vaccine. N Engl J Med 2001; 344(8): 564–72
Simonsen L, Viboud C, Elixhauser A, et al. More on RotaShield and intussusception: the role of age at the time of vaccination. J Infect Dis 2005; 192Suppl. 1: S36–43
Givon-Lavi N, Greenberg D, Dagan R. Comparison between two severity scoring scales commonly used in the evaluation of rotavirus gastroenteritis in children. Vaccine 2008; 26(46): 5798–801
Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practice (ACIP). Morb Mortal Wkly Rep 2009; 58(RR-2): 1–25
Heaton PM, Goveia MG, Miller JM, et al. Development of a pentavalent rotavirus vaccine against prevalent serotypes of rotavirus gastroenteritis. J Infect Dis 2005; 192Suppl. 1: S17–21
Kapikian AZ, Hoshino Y. To serotype or not to serotype: that is still the question. J Infect Dis 2007; 195(5): 611–4
Vesikari T, Clark HF, Offit PA, et al. Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine 2006; 24(22): 4821–9
Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354(1): 23–33
Block SL, Vesikari T, Goveia MG, et al. Efficacy, immunogenicity, and safety of a pentavalent, human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007; 119(1): 11–8
Kim DS, Lee TJ, Kang JH, et al. Immunogenicity and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy infants in Korea. Pediatr Infect Dis J 2008; 27(2): 177–8
Yen C, Jakob K, Peckham X, et al. Detection of fecal shedding of rotavirus vaccine in infants following their first dose of RotaTeq® [abstract no. 4314.7]. Pediatric Academic Societies Annual Meeting; 2009 May 2–5; Baltimore (MD)
Payne DC, Edwards KM, Bowen MD, et al. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics 2010; 125(2): e438–41
Ciarlet M, He S, Lai S, et al. Concomitant use of the 3-dose oral pentavalent rotavirus vaccine with a 3-dose primary vaccination course of a diphtheriatetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type b vaccine. Pediatr Infect Dis J 2009; 28(3): 177–81
Ciarlet M, Sani-Grosso R, Yuan G, et al. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J 2008; 27(10): 874–80
Vesikari T, Itzler R, Karvonen A, et al. RotaTeq®, a pentavalent rotavirus vaccine: efficacy and safety among infants in Europe. Vaccine 2009; 28(2): 345–51
Ciarlet M. The pentavalent rotavirus vaccine RotaTeq® [oral presentation]. 8th International Rotavirus Symposium; 2008 Jun 3–4; Istanbul
World Health Organization. Rotavirus vaccines: an update. WHO Wkly Record 2009; 84(51–52): 533–7
Clark HF, Bernstein DI, Dennehy PH, et al. Safety, efficacy, and immunogenicity of a live, quadrivalent human-bovine reassortant rotavirus vaccine in healthy infants. J Pediatr 2004; 144(2): 184–90
Vesikari T, Itzler R, Matson DO, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). Int J Infect Dis 2007; 11Suppl. 2: S29–35
Vesikari T, Karvonen A, Ferrante SA, et al. Sustained efficacy of the pentavalent rotavirus vaccine, RV5, up to 3.1 years following the last dose of vaccine. Pediatr Infect Dis J. Epub 2010 May 3
Goveia MG, Rodriguez ZM, Dallas MJ, et al. Safety and efficacy of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatr Infect Dis J 2007; 26(12): 1099–104
Goveia MG, DiNubile MJ, Dallas MJ, et al. Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr Infect Dis J 2008; 27(7): 656–8
Smith CK, McNeal MM, Meyer NR, et al. Rotavirus antigen shedding in premature infants following immunization [poster no. 1158]. 47th Annual Meeting of the Infectious Diseases Society of America; 2009 Oct 29–Nov 1; Philadelphia (PA)
Patel MM, Parashar UD. Assessing the effectiveness and public health impact of rotavirus vaccines after introduction in immunization programs. J Infect Dis 2009; 200Suppl. 1: S291–9
Cunliffe NA, Kilgore PE, Bresee JS, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull WHO 1998; 76(5): 525–37
O’Ryan ML, Hermosilla G, Osorio G. Rotavirus vaccines for the developing world. Curr Opin Infect Dis 2009; 22(5): 483–9
Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301(21): 2243–51
Dennehy PH, Goveia MG, Dallas MJ, et al. The integrated phase III safety profile of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine. Int J Infect Dis 2007; 11Suppl. 2: S36–42
Haber P, Patel M, Izurieta HS, et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States, February 1, 2006, to September 25, 2007. Pediatrics 2008; 121(6): 1206–12
Belongia EA, Irving SA, Shui IM, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J 2010; 29(1): 1–5
Centers for disease Control and Prevention. Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. Morb Mortal Wkly Rep 2010; 59(22): 687–8
Denney PH, Bertrand HR, Silas PE, et al. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008; 122(5): e1062–6
Zaman K, Sack DA, Yunus M, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine 2009; 27(9): 1333–9
Tregnaghi M, Lopez P, De Leon T, et al. Oral human rotavirus vaccine RIX4414 (Rotarix™) co-administered with routine EPI vaccinations including oral polio vaccine (OPV) is highly efficacious in Latin America [abstract no. 19.020]. 13th International Congress on Infectious Diseases; 2008 Jun 19–22; Kuala Lumpur
Dennehy PH, Brady RC, Halperin SA, et al. Comparative evaluation of safety and immunogenicity of two dosages of an oral live attenuated human rotavirus vaccine. Pediatr Infect Dis J 2005; 24(6): 481–8
Phua KB, Quak SH, Lee BW, et al. Evaluation of RIX4414, a live attenuated rotavirus vaccine, in a randomized, double blind, placebo-controlled phase 2 trial involving 2464 Singaporean infants. J Infect Dis 2005; 192Suppl. 1: S6–16
Vesikari T, Karvonen A, Korhonen T, et al. Safety and immunogenicity of RIX4414 live attenuated human rotavirus vaccine in adults, toddlers and previously uninfected infants. Vaccine 2004; 22(21–22): 2836–42
Salinas B, Perez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: a randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J 2005; 24(9): 807–16
Ruiz-Palacios GM, Guerrero ML, Bautista-Marquez A, et al. Dose response and efficacy of a live, attenuated human rotavirus vaccine in Mexican infants. Pediatrics 2007; 120(2): e253–61
Neuzil K, Madhi SA, Cunliffe N, et al. Immunogenicity of human rotavirus vaccine RIX4414 in South African and Malawian infants [abstract]. 6th World Society for Pediatric Infectious Diseases Congress; 2009 Nov 18–22; Buenos Aires
Narang A, Bose A, Pandit AN, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccines 2009; 5(6): 414–9
Moon S-S, Wang Y, Shane AL, et al. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediatr Infect Dis J. Epub 2010 May 3
Ward RL, Bernstein DI. Rotarix: a rotavirus vaccine for the world. Clin Infect Dis 2009; 48(2): 222–8
Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis 2008; 8(10): 642–9
Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354(1): 11–22
Linhares AC, Velazquez FR, Perez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371(9619): 1181–9
Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370(9601): 1757–63
Phua KB, Lim FS, Lau YL, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double blind, controlled study. Vaccine 2009; 27(43): 5936–41
Madhi SA, Cunliffe NA, Steele D, et al. Effect of rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362(4): 289–98
Neuzil K, Madhi S, Cunliffe N, et al. RIX4414 is protective against severe RVGE caused by diverse rotavirus serotypes during the first year of life in African infants [abstract]. 27 th Annual Meeting of the European Society for Pediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Madhi SA, Kirsten M, Bos P, et al. Efficacy of the human rotavirus vaccine RIX4414 against rotavirus G2P[4]/G8P[4] strains in South African infants [oral presentation]. 6th World Society for Pediatric Infectious Diseases Congress; 2009 Nov 18–22; Buenos Aires
Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for severity of diarrheal episodes. Scand J Infect Dis 1990; 22(3): 259–67
Phua KB, Lim FS, Lau YL, et al. Human rotavirus vaccine RIX4414 is highly efficacious in Asian infants during the third year of life [abstract]. 27th Annual Meeting of the European Society for Pediatric Infectious Diseases; 2009 Jun 9–13; Brussels
De Vos B, Han HH, Bouckenooghe A, et al. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr Infect Dis J 2009; 28(4): 261–6
Omenaca F, Sarlangue J, Wysocki J, et al. Immunogenicity of a rotavirus vaccine (RIX4414) in European pre-term infants with different gestational age [abstract]. 27th Annual Meeting of the European Society for Pediatric Infectious Diseases; 2009 Jun 9–13; Brussels
Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis 2007; 196(4): 537–40
Cheuvart B, Friedland LR, Abu-Elyazeed R, et al. The human rotavirus vaccine RIX4414 in infants: a review of safety and tolerability. Pediatr Infect Dis 2009; 28(3): 225–32
United States Food and Drug Administration. Update on Rotarix vaccine [online]. Available from URL: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm205539.htm [Accessed 2010 May 14]
World Health Organization. Rotavirus vaccination — WHO does not recommend any changes to use of Rotarix vaccine [media release]. 2010 Mar 10 [online]. Available from URL http://www.who.int/immunization/newsroom/news_rotavirus_vaccine_use/en/ [Accessed 2010 Mar 30]
European Medicines Agency. European Medicines Agency sees no safety concerns with the Rotarix oral vaccine [media release]. 2010 Mar 26 [online]. Available from URL: http://www.ema.europa.eu/pressoffice/emea.htm
Australian Government Department of Health and Ageing Therapeutic Goods Administration. Statement on rotavirus vaccines [media release]. 2010 May 18 [online]. Available from URL: http://www.tga.gov.au/alerts/
Tate JE, Panozzo CA, Payne DC, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics 2009; 124(2): 465–71
Panozzo CA, Tate JE, Payne DC, et al. Reduction in rotavirus after vaccine introduction: United States, 2000–2009. Morb Mortal Wkly Rep 2009; 58(41): 1146–9
Wang FT, Mast TC, Glass RJ, et al. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010; 125(2): e208–13
Curns AT, Steiner CA, Barrett M, et al. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201(11): 1617–24
Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010; 125(2): e199–207
Anderson EJ, Rupp A, Shulman ST, et al. Impact of vaccination on nosocomial transmission of rotavirus [poster no. 1149]. 47th Annual Meeting of the Infectious Diseases Society of America; 2009 Oct 29–Nov 1; Philadelphia (PA)
Lambert SB, Faux CE, Hall L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust 2009; 191(3): 157–60
Payne DC, Szilagyi PG, Staat MA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J 2009; 28(11): 948–53
Carvalho-Costa FA, Araujo IT, Santos de Assis RM, et al. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg Infect Dis 2009; 15(1): 95–7
Nakagomi T, Cuevas LE, Gurgel RG, et al. Apparent extinction of non-G2 rotavirus strains from circulation in Recife, Brazil, after introduction of rotavirus vaccine. Arch Virol 2008; 153(3): 591–3
Patel MM, de Oliveira LH, Bispo AM, et al. Rotavirus P[4]G2 in a vaccinated population, Brazil. Emerg Infect Dis 2008; 14(5): 863–5
Gurgel RG, Bohland AK, Vieira SCF, et al. Incidence of rotavirus and all-cause diarrhea in Northeast Brazil following the introduction of a national vaccination program. Gastroenterology 2009; 137(6): 1970–5
Correia JB, Patel MM, Nakagomi O, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis 2010; 201(3): 363–9
de Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ 2010; 341: c2825. doi: 10.1136/bmj.c2825
Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med 2010; 362(4): 299–305
Snelling TL, Schultz R, Graham J, et al. Rotavirus and the indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis 2009; 49(3): 428–31
Snelling TL, Andrews RM, Kirkwood CD, et al. Evaluation of the monovalent human rotavirus vaccine RIX4414 following a G2P[4] outbreak [abstract no. G1-1558b]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco (CA)
Giaquinto C, Van Damme P, Huet F, et al., on behalf of the RSG. Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL Study. J Infect Dis 2007; 195Suppl. 1: S36–44
Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modelling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices — modelling studies. Value Health 2003; 6(1): 9–17
WHO Commission on Macroeconomics and Health. Report on macroeconomics and health: investing in health for economic development, 2001 [online]. Available from URL: http://www.stluciauhc.org/res/25PDF [Accessed 2010 Jan]
Beutels P, Scuffham PA, MacIntyre CR. Funding of drugs: do vaccines warrant a different approach? Lancet Infect Dis 2008; 8(11): 727–33
Bilcke J, Beutels P. Reviewing the cost effectiveness of rotavirus vaccination: the importance of uncertainty in the choice of data sources. Pharmacoeconomics 2009; 27(4): 281–97
Jit M, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales, part II: the potential cost effectiveness of vaccination. Vaccine 2007; 25(20): 3971–9
Newall AT, Beutels P, Macartney K, et al. The cost-effectiveness of rotavirus vaccination in Australia. Vaccine 2007; 25(52): 8851–60
Martin A, Batty A, Roberts JA, et al. Cost-effectiveness of infant vaccination with RIX4414 (Rotarix) in the UK. Vaccine 2009; 27(33): 4520–8
Milne RJ, Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value Health 2009; 12(6): 888–98
Curns AT, Coffin F, Glasser JW, et al. Projected impact of the new rotavirus vaccination program on hospitalizations for gastroenteritis and rotavirus disease among US children <5 years of age during 2006–2015. J Infect Dis 2009; 200Suppl. 1: S49–56
Atherly D, Dreibelbis R, Parashar UD, et al. Rotavirus vaccination: cost-effectiveness and impact on child mortality in developing countries. J Infect Dis 2009; 200Suppl. 1: S28–38
Milne RJ, Grimwood K. Should rotavirus vaccines be included in the national immunization program of a small developed country? Exp Rev Pharmacoeconomics Res 2009; 9(5): 401–4
World Health Organization. Rotavirus vaccination. WHO Wkly Record 2009; 84(23): 232–6
Vesikari T, Karvonen A, Forrest BD, et al. Neonatal administration of rhesus rotavirus tetravalent vaccine. Pediatr Infect Dis J 2006; 25(2): 118–22
Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348(18): 1737–46
Han HH, Suryakiran PV, Debrus S, et al. Importance of early protection by vaccinating with a human rotavirus vaccine RIX4414 [oral presentation]. 6th World Society for Pediatric Infectious Diseases Congress; 2009 Nov 18–22; Buenos Aires
Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009; 373(9674): 1543–9
Naghipour M, Nakagomi T, Nalagomi O. Issues with reducing the rotavirusassociated mortality by vaccination in developing countries. Vaccine 2008; 26(26): 3236–41
Melliez H, Boelle PY, Baron S, et al. Effectiveness of childhood vaccination against rotavirus in sub-Saharan Africa: the case of Nigeria. Vaccine 2007; 25(2): 298–305
Grimwood K, Bines JE. Rotavirus vaccines must perform in low-income countries too. Lancet 2007; 370(9601): 1739–40
Patel MM, Clark AD, Glass RI, et al. Broadening the age restriction for initiating rotavirus vaccination in regions with high rotavirus mortality: benefits of mortality reduction versus risk of fatal intussusception. Vaccine 2009; 27(22): 2916–22
Grimwood K, Kirkwood CD. Human rotavirus vaccines: too early for the strain to tell. Lancet 2008; 371(9619): 1144–5
Munos MK, Fischer Walker CL, Black RE. The effect of rotavirus vaccine on diarrhoea mortality. Int J Epidemiol 2010; 39(2): i56–62
Wardlaw T, Salama P, Brocklehurst C, et al. Diarrhoea: why children are still dying and what can be done. Lancet 2010; 375(9718): 870–2
Acknowledgements
There was no funding for the preparation of this manuscript. Keith Grimwood has been a member of a Rotavirus Advisory Board and received support for conference attendance, lecture fees, and a research grant from GlaxoSmithKline; he has also received a research grant from Merck. Stephen Lambert has been a co-investigator of vaccine trials and epidemiological studies sponsored by Merck, CSL Ltd, and GlaxoSmithKline. He has received support for conference attendance from GlaxoSmithKline and CSL Ltd. Richard Milne has received funding for an economic evaluation and support for conference attendance from Merck.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimwood, K., Lambert, S.B. & Milne, R.J. Rotavirus Infections and Vaccines. Pediatr-Drugs 12, 235–256 (2010). https://doi.org/10.2165/11537200-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11537200-000000000-00000