Abstract
Objective
To evaluate the cost effectiveness and cost utility of the use of valsartan in addition to standard therapy for the treatment of patients with chronic heart failure with low left ventricular ejection fraction (LVEF).
Methods
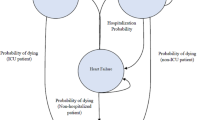
The study was conducted by means of a cohort simulation based on a probabilistic Markov model and projecting the 23-month follow-up results of the Val-HeFT (Valsartan Heart Failure Trial) study over a 10-year time horizon. The model included four states (New York Heart Association [NYHA] classes II, III, IV, and death), and had a cycle duration of 1 month. Probabilistic simulations were performed using the WinBUGS software for Bayesian analysis. The distribution of patient parameters (sex, age, use of β-adrenoceptor antagonists, and ACE inhibitors) in the simulated population were derived from the Italian heart failure patient population. Individual mortality data were derived from general mortality data by multiplying by a NYHA state-specific relative risk, while the probability of changing NYHA class was taken from the Val-HeFT data. Costs (2007 values) were calculated from the perspective of the Italian Health Service (IHS) and included costs for drugs and heart failure hospitalizations. Quality-of-life (QOL) weights were obtained byusing published health-related QOL data for heart failure patients.A 3.5% annual discount rate was applied. Probabilistic sensitivity analysis was performed on each parameter using original-source 95% confidence interval (CI) values, or a ±10% range when 95% CI values were unavailable.
Results
For the 10-year time horizon, patients were estimated to live for an average of 2.3 years or 1.7 quality-adjusted life-years (QALYs), with slight increases in the valsartan group. In this group, hospitalizations for worsening heart failure were predicted to be significantly reduced and overall treatment costs per patient to decrease by about €550. In subgroup analyses, valsartan lost dominance in patients in NYHA II, and in those receiving β-adrenoceptor antagonists or ACE inhibitors; the mean incremental cost-utility ratio for these groups was 21 240, 129 200, and 36 500 €/QALY, respectively.
Conclusions
Valsartan in addition to standard therapy is predicted to dominate standard therapy alone in Italian patients with mild to severe heart failure and low LVEF. There are relevant differences among various patient subgroups, and valsartan is expected to be good value for money particularly in the treatment of the most severe and less intensively treated (no ACE inhibitors, no β-adrenoceptor antagonist) heart failure patients.











Similar content being viewed by others
References
Consensus conference on the management of heart failure [in Italian]. G Ital Cardiol (Rome) 2006; 7 (6): 387–432.
National Institute for Clinical Excellence. Chronic heart failure: management of chronic heart failure in adults in primary and secondary care [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/Full_HF_Guideline.pdf [Accessed 2007 Dec 14].
ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008 Oct; 29: 2388–442.
Hunt SA, on behalf of the American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005 Sep 20; 46(6): e1–82.
Julius S, Kjeldsen SE, Weber M, et al., VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363: 2022–31.
Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both [published erratum appears in N Engl J Med 2004 Jan 8; 350 (2): 203]. N Engl J Med 2003 Nov 13; 349(20): 1893–906.
Cohn JN, Tognoni G, on behalf of the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–75.
Carson P, Tognoni G, Cohn JN. Effect of valsartan on hospitalization: results from Val-HeFT. J Card Fail 2003; 9: 164–71.
Reed SD, Friedman JY, Velazquez EJ, et al. Multinational economic evaluation of valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J 2004; 148: 122–8.
Smith DG, Cerulli A, Frech FH. Use of valsartan for the treatment of heartfailure patients not receiving ACE inhibitors: a budget impact analysis. Clin Ther 2005; 27: 951–9.
Krumholz HM, Parent EM, Tu N, et al. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med 1997; 157: 99–104.
Gooding J, Jette AM. Hospital readmissions among the elderly. J Am Geriatr Soc 1985; 33: 596–601.
McMurray J, Hart W, Rhodes G. An evaluation of the cost of heart failure to the National Health Service in the UK. Br Med Econ 1993; 6: 99–110.
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993 Oct–Dec; 13(4): 322–38.
Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making 1983; 3: 419–58.
Kuntz KM, Weinstein MC. Modelling in economic evaluations. In: Drummond M, McGuire A, editors. Economic evaluation in health care. Oxford: Oxford University Press, 2001: 146–53.
Caro JJ. Pharmacoeconomic analyses using discrete even simulation. Pharmacoeconomics 2005; 23(4): 323–32.
The Bugs Project: winbugs [online]. Available from URL: http://www.mrc-bsu.cam.ac.uk/bugs/winbugs/contents.shtml [Accessed 2009 Jun 8].
Cooper NJ, Sutton AJ, Abrams KR. Decision analytical economic modelling within a Bayesian framework: application to prophylactic antibiotics use for caesarean section. Stat Methods Med Res 2002; 11: 491–512.
Cooper NJ, Sutton AJ, Abrams KR, et al. Comprehensive decision analytical modelling in economic evaluation: a Bayesian approach. Health Econ 2004; 13: 203–26.
Spiegelhalter DJ, Best NG. Bayesian approaches to multiple sources of evidence and uncertainty in complex cost/effectiveness modelling. Stat Med 2003; 22: 3687–709.
Iannazzo S. Le tecniche statistiche bayesiane e la loro applicazione in modelli di simulazione probabilistica. Farmeconomia e percorsi terapeutici 2007; 8(1): 5–13.
Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in healthcare evaluation: report of the ISPOR task force on good research practices — modeling studies. Value Health 2003; 6: 9–17.
Hobbs FD, Kenkre JE, Roalfe AK, et al. Impact of heart failure and left ventricular systolic dysfunction on quality of life. Eur Heart J 2002; 23: 1867–76.
Davies MK, Hobbs FDR, Davis RC, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001; 358: 439–44.
Nichol MB, Sengupta N, Globe DR. Evaluating quality-adjusted life years: estimation of the Health Utility Index (HUI2) from the SF-36. Med Decis Making 2001; 21: 105–112.
SF-36.org. SF-36® PCS, MCS and NBS calculator [online]. Available from URL: http://www.sf-36.org/nbscalc/index.shtml [Accessed 2007 Dec 14].
ISTAT. Annuario Statistico Italiano 2007. Rome: ISTAT, 2007.
Il database dell’Italian Network on Congestive Heart Failure (IN-CHF): risultati e prospettive. Atti del Convegno. Naples: 24 giugno, 1999.
L’Informatore Farmaceutico. Aggiornamento novembre — dicembre 2007. Milan: Elsevier Masson, 2007.
Ministero della Salute. DECRETO 12 settembre 2006. Ricognizione e primo aggiornamento delle tariffe massime per la remunerazione delle prestazioni sanitarie. (GU n. 289 del 13-12-2006- Suppl. Ordinario n.234).
Morsanutto A, Mantovani L, Ros B, et al. Costs and outcomes after first heart failure hospital admission: a longitudinal study using administrative databases [abstract PCV27]. Value Health 2005; 8(6): A96.
National Institute for Clinical Excellence. Updated guide to the methods of technology appraisal: June 2008 [online]. Available from URL: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf [Accessed 2007 Dec 14].
ISTAT. Popolazione residente al 1 gennaio 2006 [online]. Available from URL: http://demo.istat.it/pop2006/index.html [Accessed 2007 Dec 14].
Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation: a practical approach. Med Decis Making 1985; 5: 157–77.
Briggs AH. Handling uncertainty in economic evaluations and presenting the results. In: Drummond M, McGuire A, editors. Economic evaluation in health care. Oxford: Oxford University Press, 2001: 172–214.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold: what it is and what that means. Pharmacoeconomics 2008; 26(9): 733–44.
Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 2004; 13: 437–52.
Acknowledgments
This study was funded by an unrestricted research grant from Novartis Farma, Origgio (VA), Italy. All authors have worked as external consultants for Novartis. Publication of the results of the study was not contingent on the sponsor’s approval. The sponsor had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, nor in the preparation, review, or approval of the manuscript. L. Pradelli has also received consultancies and/or research grants from Roche, GlaxoSmithKline, and Amgen, among other pharmaceutical companies, and S. Iannazzo has also received consultancy and research grants from Roche, GlaxoSmithKline, and Amgen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pradelli, L., Iannazzo, S. & Zaniolo, O. The Cost Effectiveness and Cost Utility of Valsartan in Chronic Heart Failure Therapy in Italy. Am J Cardiovasc Drugs 9, 383–392 (2009). https://doi.org/10.2165/11315730-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11315730-000000000-00000