Abstract
Background and Objective: Increased antibacterial use is associated with a greater likelihood of reduced effectiveness as a result of resistance development in the future. The objective of this study was to evaluate the cost effectiveness of ertapenem versus piperacillin/tazobactam in the treatment of diabetic foot infections (DFIs) from the UK NHS perspective, accounting for the development of antibacterial resistance over time.
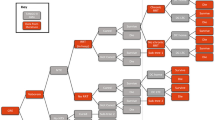
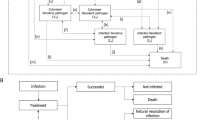
Methods: A decision-tree model was developed to estimate the cost effectiveness of ertapenem versus piperacillin/tazobactam at different timepoints in the 36 months following introduction of treatment. Development of antibacterial resistance was incorporated in the analysis using a previously published compartment (susceptible-infected-susceptible) model. The development of resistance was a function of the clearance rate of pathogens and the size of the proportion of the population prescribed the antibacterial. The microbiological clearance rate and clinical success rates were assumed to be related and were obtained from the SIDESTEP study. These data included resistant pathogens (either acquired or intrinsic resistance) such as Enterobacteriaceae, meticillin-resistant Staphylococcus aureus, enterococci and Pseudomonas aeruginosa.
Model outcomes over time included lifetime QALYs, directmedical costs (year 2006 values) and cost per QALY saved. Clinical efficacy of second-line treatment, direct medical costs and utilities were derived from other existing studies. Probabilistic sensitivity analyses were undertaken to estimate the uncertainty of model outcomes. Costs and QALYs were discounted at 3.5% per annum.
Results: The model suggested savings of £407 (95% uncertainty interval [UI] −337, 1501) per patient when DFIs were treated with ertapenem instead of piperacillin/tazobactam after 1 month’s treatment. Probabilistic sensitivity analysis suggested a 91% probability of the incremental cost per QALY saved being within a threshold for cost effectiveness of £20 000. After 3 years it is expected that the antibacterial resistance profile with piperacillin/tazobactam would increase at a greater rate than with ertapenem. As a result, the cost savings with ertapenem are expected to increase to £3465 (95% UI 2502, 4564), and ertapenem will additionally result in greater clinical success rates and lifetime QALY savings (1.16; 95% UI 0.46, 2.06).
Conclusion: Ertapenem appears to be a cost-saving and possibly an economically dominant therapy over piperacillin/tazobactam for the treatment of patients with DFIs from the UK NHS perspective.








Similar content being viewed by others
References
Lipsky BA. A report from the international consensus on diagnosing and treating the infected diabetic foot. Diabetes Metab Res Rev 2004; 20 Suppl. 1: S68–77
Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003; 361: 1545–51
Tennvall GR, Apelqvist J, Eneroth M. Costs of deep foot infections in patients with diabetes mellitus. Pharmacoeconomics 2000; 18 (3): 225–38
Ramsey SD, Newton K, Blough D, et al. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22: 382–7
Lipsky BA, Berendt AR, Deery HG, et al. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004; 39: 885–910
Reiber GE. The epidemiology of diabetic foot problems. Diabet Med 1996; 13 Suppl. 1: S6–11
Armstrong DG, Liswood PJ, Todd WF. Prevalence of mixed infections in the diabetic pedal wound: a retrospective review of 112 infections. J Am Podiatr Med Assoc 1995; 85: 533–7
Calhoun JH, Cantrell J, Cobos J, et al. Treatment of diabetic foot infections: Wagner classification, therapy, and outcome. Foot Ankle 1988; 9: 101–6
Lipsky BA, Armstrong DG, Citron DM, et al. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): prospective, randomised, controlled, doubleblinded, multicentre trial. Lancet 2005; 366: 1695–703
McGowan JE. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Infect Dis 1983; 5: 1033–48
Cohen FL, Tartasky D. Microbial resistance to drug therapy: a review. Amer J Infection Control 1997; 25: 51–64
Hanberger H, Hoffmann M, Lindgren S, et al. High incidence of antibiotic resistance among bacteria in four intensive care units at a university hospital in Sweden. Scand J Infect Dis 1997; 29: 607–14
Muder RR, Brennen C, Drenning SD, et al. Multiply antibioticresistant gram-negative bacilli in a long-term-care facility: a case control study of patient risk factors and prior antibiotic use. Infect Control Hosp Epidemiol 1997; 18 (12): 809–13
Babini GS, Livermore DM. Antimicrobial resistance amongst Klebsiella spp. collected from intensive care units in Southern and Western Europe in 19971998. J Antimicrob Chemother 2000; 45: 183–9
Paterson DL, Ko WC, Von Gottberg A, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol 2001; 39: 2206–12
DiNubile MJ, Friedland I, Chan CY, et al. Bowel colonization with resistant Gram-negative bacilli after antimicrobial therapy of intra-abdominal infections: observations from two randomized comparative clinical trials of ertapenem therapy. Eur J Clin Microbiol Infect Dis 2005; 24: 443–9
Laxminarayan R, Brown GM. Economics of antibiotic resistance: a theory of optimal use. J Environ Econ Management 2001; 42: 183–206
Grayson ML, Gibbons GW, Habershaw GM, et al. Use of ampicillin/sulbactam versus imipenem/cilastatin in the treatment of limb-threatening foot infections in diabetic patients. Clin Infect Dis 1994; 18: 683–93
British National Formulary. London: British Medical Association and Royal Pharmaceutical Society of Great Britain, 2006
UK Department of Health. NHS reference cost, 2003 and national tariff 2004 (payment by results core tools 2004) [online]. Available from URL: (http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4070195) [Accessed 2006 Oct 5]
NHS Trust and PCT combined reference cost, 2005 [online]. Available from URL: (http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4133221) [Accessed 2006 Oct 5]
UK Government Actuary Department 2002-2004. Available from URL: (http://www.gad.gov.uk/Life_Tables/docs/) [Accessed 2006 Oct 1]
Sullivan S, Lew D, Devine E, et al. Health state preference assessment in diabetic peripheral neuropathy. Pharmacoeconomics 2002; 20 (15): 1079–89
Nelson EA, O’Meara S, Craig D, et al. A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess 2006; 10: iii-iv, ix-x, 1–221
Acknowledgements
This study was funded by Merck & Co, which manufactures ertapenem. Ritesh Kumar is an employee of Merck & Co., and is therefore eligible to receive stock options in the company. Yehuda Carmeli has received consultancies, honoraria and grants from Merck & Co.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jansen, J.P., Kumar, R. & Carmeli, Y. Accounting for the Development of Antibacterial Resistance in the Cost Effectiveness of Ertapenem versus Piperacillin/Tazobactam in the Treatment of Diabetic Foot Infections in the UK. Pharmacoeconomics 27, 1045–1056 (2009). https://doi.org/10.2165/11310080-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11310080-000000000-00000