Abstract
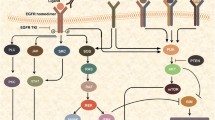
The epidermal growth factor receptor (EGFR) is over-expressed, as well as mutated, in many types of cancers. In particular, the EGFR variant type III mutant (EGFRvIII) has attracted much attention as it is frequently and exclusively found on many tumor cells, and hence both EGFR and EGFRvIII have been proposed as valid targets in many cancer therapy settings. Different strategies have been developed in order to either inhibit EGFR/EGFRvIII activity or to ablate EGFR/EGFRvIII-positive tumor cells. Drugs that inhibit these receptors include monoclonal antibodies (mAbs) that bind to the extracellular part of EGFR, blocking the binding sites for the EGFR ligands, and intracellular tyrosine kinase inhibitors (TKIs) that block the ATP binding site of the tyrosine kinase domain. Besides an EGFRvIII-targeted vaccine, conjugated anti-EGFR mAbs have been used in different settings to deliver lethal agents to the EGFR/EGFRvIII-positive cells; among these are radio-labelled mAbs and immunotoxins. This article reviews the current status and efficacy of EGFR/EGFRvIII-targeted therapies.





Similar content being viewed by others
References
Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors 2008 Oct; 26 (5): 263–74
Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 2005 May; 5 (5): 341–54
Ullrich A, Coussens L, Hayflick JS, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984; 309 (5967): 418–25
Cohen S, Ushiro H, Stoscheck C, et al. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem 1982 Feb; 257 (3): 1523–31
Lax I, Johnson A, Howk R, et al. Chicken epidermal growth factor (EGF) receptor: cDNA cloning, expression in mouse cells, and differential binding of EGF and transforming growth factor alpha. Mol Cell Biol 1988 May; 8 (5): 1970–8
Lax I, Burgess WH, Bellot F, et al. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol 1988 Apr; 8 (4): 1831–4
Carpenter G, Lembach KJ, Morrison MM, et al. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem 1975 Jun; 250 (11): 4297–304
Marquardt H, Hunkapiller MW, Hood LE, et al. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science 1984 Mar; 223 (4640): 1079–82
Shoyab M, Plowman GD, McDonald VL, et al. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science 1989 Feb; 243 (4894 Pt 1): 1074–6
Lax I, Bellot F, Howk R, et al. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J 1989 Feb; 8 (2): 421–7
Watanabe T, Shintani A, Nakata M, et al. Recombinant human betacellulin: molecular structure, biological activities, and receptor interaction. J Biol Chem 1994 Apr; 269 (13): 9966–73
Komurasaki T, Toyoda H, Uchida D, et al. Epiregulin binds to epidermal growth factor receptor and ErbB-4 and induces tyrosine phosphorylation of epidermal growth factor receptor, ErbB-2, ErbB-3 and ErbB-4. Oncogene 1997 Dec; 15 (23): 2841–8
Higashiyama S, Abraham JA, Miller J, et al. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 1991 Feb; 251 (4996): 936–9
Bajaj M, Waterfield MD, Schlessinger J, et al. On the tertiary structure of the extracellular domains of the epidermal growth factor and insulin receptors. Biochim Biophys Acta 1987 Nov; 916 (2): 220–6
Weber W, Bertics PJ, Gill GN. Immunoaffinity purification of the epidermal growth factor receptor: stoichiometry of binding and kinetics of self-phosphorylation. J Biol Chem 1984 Dec; 259 (23): 14631–6
Zhang X, Gureasko J, Shen K, et al. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006 Jun; 125 (6): 1137–49
Ferguson KM. Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys 2008; 37: 353–73
Lu C, Mi LZ, Grey MJ, et al. Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Mol Cell Biol 2010 Nov; 30 (22): 5432–43
Chung I, Akita R, Vandlen R, et al. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 2010 Apr; 464 (7289): 783–7
Stamos J, Sliwkowski MX, Eigenbrot C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J Biol Chem 2002 Nov; 277 (48): 46265–72
Honegger AM, Dull TJ, Felder S, et al. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell 1987 Oct; 51 (2): 199–209
Honegger AM, Szapary D, Schmidt A, et al. A mutant epidermal growth factor receptor with defective protein tyrosine kinase is unable to stimulate proto-oncogene expression and DNA synthesis. Mol Cell Biol 1987 Dec; 7 (12): 4568–71
Chen WS, Lazar CS, Poenie M, et al. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. Nature 1987 Aug; 38 (6133): 820–3
Reinehr R, Haussinger D. Epidermal growth factor receptor signaling in liver cell proliferation and apoptosis. Biol Chem 2009 Oct; 390 (10): 1033–7
Schneider MR, Sibilia M, Erben RG. The EGFR network in bone biology and pathology. Trends Endocrinol Metab 2009 Dec; 20 (10): 517–24
Wong RW. Transgenic and knock-out mice for deciphering the roles of EGFR ligands. Cell Mol Life Sci 2003 Jan; 60 (1): 113–8
Zandi R, Larsen AB, Andersen P, et al. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal 2007 Oct; 19 (10): 2013–23
Herbst RS, Langer CJ. Epidermal growth factor receptors as a target for cancer treatment: the emerging role of IMC-C225 in the treatment of lung and head and neck cancers. Semin Oncol 2002 Feb; 29 (1 Suppl. 4): 27–36
Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer 2002 Mar; 94 (5): 1593–611
Petrides PE, Bock S, Bovens J, et al. Modulation of pro-epidermal growth factor, pro-transforming growth factor alpha and epidermal growth factor receptor gene expression in human renal carcinomas. Cancer Res 1990 Jul; 50 (13): 3934–9
Yao M, Shuin T, Misaki H, et al. Enhanced expression of c-myc and epidermal growth factor receptor (C-erbB-1) genes in primary human renal cancer. Cancer Res 1988 Dec; 48 (23): 6753–7
Hirsch FR, Scagliotti GV, Langer CJ, et al. Epidermal growth factor family of receptors in preneoplasia and lung cancer: perspectives for targeted therapies. Lung Cancer 2003 Aug; 41Suppl. 1: S29–42
Ge H, Gong X, Tang CK. Evidence of high incidence of EGFRvIII expression and coexpression with EGFR in human invasive breast cancer by laser capture microdissection and immunohistochemical analysis. Int J Cancer 2002 Mar; 98 (3): 357–61
Wong AJ, Bigner SH, Bigner DD, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A 1987 Oct; 84 (19): 6899–903
Hatanpaa KJ, Burma S, Zhao D, et al. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia 2010 Sep; 12 (9): 675–84
Merlino GT, Ishii S, Whang-Peng J, et al. Structure and localization of genes encoding aberrant and normal epidermal growth factor receptor RNAs from A431 human carcinoma cells. Mol Cell Biol 1985 Jul; 5 (7): 1722–34
Batra SK, Castelino-Prabhu S, Wikstrand CJ, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ 1995 Oct; 6 (10): 1251–9
Sugawa N, Ekstrand AJ, James CD, et al. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci U S A 1990 Nov; 87 (21): 8602–6
Moscatello DK, Holgado-Madruga M, Godwin AK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res 1995 Dec; 55 (23): 5536–9
Wikstrand CJ, Reist CJ, Archer GE, et al. The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol 1998 Apr; 4 (2): 148–58
Okamoto I, Kenyon LC, Emlet DR, et al. Expression of constitutively activated EGFRvIII in non-small cell lung cancer. Cancer Sci 2003 Jan; 94 (1): 50–6
Tang CK, Gong XQ, Moscatello DK, et al. Epidermal growth factor receptor vIII enhances tumorigenicity in human breast cancer. Cancer Res 2000 Jun; 60 (11): 3081–7
Olapade-Olaopa EO, Moscatello DK, MacKay EH, et al. Evidence for the differential expression of a variant EGF receptor protein in human prostate cancer. Br J Cancer 2000 Jan; 82 (1): 186–94
Sok JC, Coppelli FM, Thomas SM, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res 2006 Sep; 12 (17): 5064–73
Garcia de Palazzo IE, Adams GP, Sundareshan P, et al. Expression of mutated epidermal growth factor receptor by non-small cell lung carcinomas. Cancer Res 1993 Jul; 53 (14): 3217–20
Wikstrand CJ, McLendon RE, Friedman AH, et al. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res 1997 Sep; 57 (18): 4130–40
Morinaga R, Okamoto I, Fujita Y, et al. Association of epidermal growth factor receptor (EGFR) gene mutations with EGFR amplification in advanced non-small cell lung cancer. Cancer Sci 2008 Dec; 9 (12): 2455–60
Ekstrand AJ, James CD, Cavenee WK, et al. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res 1991 Apr; 51 (8): 2164–72
Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 2005 Feb; 11 (4): 1462–6
Liu TF, Tatter SB, Willingham MC, et al. Growth factor receptor expression varies among high-grade gliomas and normal brain: epidermal growth factor receptor has excellent properties for interstitial fusion protein therapy. Mol Cancer Ther 2003 Aug; 2 (8): 783–7
Hall WA, Merrill MJ, Walbridge S, et al. Epidermal growth factor receptors on ependymomas and other brain tumors. J Neurosurg 1990 Apr; 72 (4): 641–6
Heimberger AB, Suki D, Yang D, et al. The natural history of EGFR and EGFRvIII in glioblastoma patients. J Transl Med 2005 Oct 19; 3: 38
Agero AL, Dusza SW, Benvenuto-Andrade C, et al. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006 Oct; 55 (4): 657–70
Kawamoto T, Sato JD, Le A, et al. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci U S A 1983 Mar; 80 (5): 1337–41
Goldstein NI, Prewett M, Zuklys K, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res 1995 Nov; 1 (11): 1311–8
Li S, Schmitz KR, Jeffrey PD, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005 Apr; 7 (4): 301–11
Jaramillo ML, Leon Z, Grothe S, et al. Effect of the anti-receptor ligand-blocking 225 monoclonal antibody on EGF receptor endocytosis and sorting. Exp Cell Res 2006 Sep 10; 312 (15): 2778–90
Kurai J, Chikumi H, Hashimoto K, et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res 2007 Mar; 13 (5): 1552–61
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004 Jul; 351 (4): 337–45
Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009 Apr; 360 (14): 1408–17
Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011 May; 29 (15): 2011–9
Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009 Feb; 27 (5): 663–71
Bokemeyer C, Bondarenko I, Hartmann JT, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011 Jul; 22 (7): 1535–46
Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006 Feb; 354 (6): 567–78
Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 2007 Jun; 25 (16): 2171–7
Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008 Sep; 359 (11): 1116–27
Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010 Feb; 28 (6): 911–7
Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009 May; 373 (9674): 1525–31
Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol 2010 May; 12 (5): 508–16
Yang XD, Jia XC, Corvalan JR, et al. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res 1999 Mar; 59 (6): 1236–43
Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007 May; 25 (13): 1658–64
Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008 Apr; 26 (10): 1626–34
Van Cutsem E, Siena S, Humblet Y, et al. An open-label, single-arm study assessing safety and efficacy of panitumumab in patients with metastatic colorectal cancer refractory to standard chemotherapy. Ann Oncol 2008 Jan; 19 (1): 92–8
Douillard JY, Salvatore S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28 (31): 4697–705
Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009 Feb; 27 (5): 672–80
Lammerts van Bueren JJ, Bleeker WK, Bogh HO, et al. Effect of target dynamics on pharmacokinetics of a novel therapeutic antibody against the epidermal growth factor receptor: implications for the mechanisms of action. Cancer Res 2006 Aug; 66 (15): 7630–8
Lammerts van Bueren JJ, Bleeker WK, Brannstrom A, et al. The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility. Proc Natl Acad Sci U S A 2008 Apr; 105 (16): 6109–14
Bleeker WK, Lammerts van Bueren JJ, van Ojik HH, et al. Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol 2004 Oct; 173 (7): 4699–707
Machiels JP, Subramanian S, Ruzsa A, et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol 2011 Apr; 12 (4): 333–43
Li S, Kussie P, Ferguson KM. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8. Structure 2008 Feb; 16 (2): 216–27
Dienstmann R, Tabernero J. Necitumumab, a fully human IgG1 mAb directed against the EGFR for the potential treatment of cancer. Curr Opin Investig Drugs 2010 Dec; 11 (12): 1434–41
Kuenen B, Witteveen PO, Ruijter R, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res 2010 Mar; 16 (6): 1915–23
Dienstmann R, Felip E. Necitumumab in the treatment of advanced non-small cell lung cancer: translation from preclinical to clinical development. Expert Opin Biol Ther 2011 Sep; 11 (9): 1223–31
Murthy U, Basu A, Rodeck U, et al. Binding of an antagonistic monoclonal antibody to an intact and fragmented EGF-receptor polypeptide. Arch Biochem Biophys 1987 Feb; 252 (2): 549–60
Faillot T, Magdelenat H, Mady E, et al. A phase I study of an anti-epidermal growth factor receptor monoclonal antibody for the treatment of malignant gliomas. Neurosurgery 1996 Sep; 39 (3): 478–83
Wersall P, Fagerberg J, Ohlsson I, et al. Induction of a T- and B-cell response against a unique amino acid sequence of the mouse IgG2A hinge region in a MAb-treated patient. Int J Cancer 1997 Dec; 73 (6): 790–4
Wersall P, Ohlsson I, Biberfeld P, et al. Intratumoral infusion of the monoclonal antibody, mAb 425, against the epidermal-growth-factor receptor in patients with advanced malignant glioma. Cancer Immunol Immunother 1997 May; 44 (3): 157–64
Schmiedel J, Blaukat A, Li S, et al. Matuzumab binding to EGFR prevents the conformational rearrangement required for dimerization. Cancer Cell 2008 Apr; 13 (4): 365–73
Takeda Pharmaceutical Company Limited. Takeda discontinues development of matuzumab [media release]. 2008 Feb 18 [online]. Available from URL: http://www.takeda.com/press/article_29042.html [Accessed 2012 Feb 1]
Boland W, Bebb G. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer. Biologics 2010; 4: 289–98
Mateo C, Moreno E, Amour K, et al. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology 1997 Mar; 3 (1): 71–81
Diaz Miqueli A, Blanco R, Garcia B, et al. Biological activity in vitro of anti-epidermal growth factor receptor monoclonal antibodies with different affinities. Hybridoma (Larchmt) 2007 Dec; 26 (6): 423–31
Choi HJ, Sohn JH, Lee CG, et al. A phase I study of nimotuzumab in combination with radiotherapy in stages IIB-IV non-small cell lung cancer unsuitable for radical therapy: Korean results. Lung Cancer 2011 Jan; 71 (1): 55–9
Strumberg D, Schultheis B, Scheulen ME, et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs. Epub 2010 Dec 21
Bebb G, Smith C, Rorke S, et al. Phase I clinical trial of the anti-EGFR monoclonal antibody nimotuzumab with concurrent external thoracic radiotherapy in Canadian patients diagnosed with stage IIb, III or IV non-small cell lung cancer unsuitable for radical therapy. Cancer Chemother Pharmacol 2011 Apr; 67 (4): 837–45
You B, Brade A, Magalhaes JM, et al. A dose-escalation phase I trial of nimotuzumab, an antibody against the epidermal growth factor receptor, in patients with advanced solid malignancies. Invest New Drugs 2011 Oct; 29 (5): 996–1003
Garrido G, Tikhomirov IA, Rabasa A, et al. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile. Cancer Biol Ther 2011 Feb; 11 (4): 373–82
Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Research 2010 Jan; 70 (2): 588–97
Skartved NJ, Jacobsen HJ, Pedersen MW, et al. Preclinical pharmacokinetics and safety of Sym004: a synergistic antibody mixture directed against epidermal growth factor receptor. Clin Cancer Res 2011 Sep; 17 (18): 5962–72
Mamot C, Drummond DC, Greiser U, et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res 2003 Jun; 63 (12): 3154–61
Mamot C, Ritschard R, Kung W, et al. EGFR-targeted immunoliposomes derived from the monoclonal antibody EMD72000 mediate specific and efficient drug delivery to a variety of colorectal cancer cells. J Drug Target 2006 May; 14 (4): 215–23
Yang L, Mao H, Wang YA, et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small 2009 Feb; 5 (2): 235–43
Brady LW, Markoe AM, Woo DV, et al. Iodine125 labeled anti-epidermal growth factor receptor-425 in the treatment of malignant astrocytomas: a pilot study. J Neurosurg Sci 1990 Jul–Dec; 34 (3–4): 243–9
Li L, Quang TS, Gracely EJ, et al. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg 2010 Aug; 113 (2): 192–8
Drexel University. Access protocol for MAB-425 radiolabeled with I-125 for high grade gliomas [ClinicalTrials.gov identifier NCT01317888]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Feb 1]
Torres LA, Perera A, Batista JF, et al. Phase I/II clinical trial of the humanized anti-EGF-r monoclonal antibody h-R3 labelled with 99mTc in patients with tumour of epithelial origin. Nucl Med Commun 2005 Dec; 26 (12): 1049–57
Casaco A, Lopez G, Garcia I, et al. Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188 Re in adult recurrent high-grade glioma. Cancer Biol Ther 2008 Mar; 7 (3): 333–9
Crombet T, Torres L, Neninger E, et al. Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother 2003 Mar–Apr; 26 (2): 139–48
Crombet T, Torres O, Rodriguez V, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma 2001 Apr; 20 (2): 131–6
Crombet T, Torres O, Neninger E, et al. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor. Cancer Biother Radiopharm 2001 Feb; 16 (1): 93–102
Allen C, Vongpunsawad S, Nakamura T, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res 2006 Dec; 66 (24): 11840–50
Phuong LK, Allen C, Peng KW, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res 2003 May; 63 (10): 2462–9
Myers R, Harvey M, Kaufmann TJ, et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum Gene Ther 2008 Jul; 19 (7): 690–8
Paraskevakou G, Allen C, Nakamura T, et al. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol Ther 2007 Apr; 15 (4): 677–86
Mayo Clinic. Viral therapy in treating patients with recurrent glioblastoma multiforme [ClinicalTrials.gov identifier NCT00390299]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Feb 1]
Mathew M, Verma RS. Humanized immunotoxins: a new generation of immunotoxins for targeted cancer therapy. Cancer Sci 2009 Aug; 100 (8): 1359–65
Pastan I. Targeted therapy of cancer with recombinant immunotoxins. Biochim Biophys Acta 1997 Oct; 1333 (2): C1–6
Pastan I, Hassan R, Fitzgerald DJ, et al. Immunotoxin therapy of cancer. Nat Rev Cancer 2006 Jul; 6 (7): 559–65
Kounnas MZ, Morris RE, Thompson MR, et al. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J Biol Chem 1992 Jun; 267 (18): 12420–3
Ogata M, Chaudhary VK, Pastan I, et al. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. J Biol Chem 1990 Nov; 265 (33): 20678–85
Iglewski BH, Liu PV, Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun 1977 Jan; 15 (1): 138–44
Duke University. Study of Immunotoxin, MR1-1 [ClinicalTrials.gov identifier NCT01009866]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://www.clinicaltrials.gov [Accessed 2012 Feb 1]
Kuan CT, Wikstrand CJ, Archer G, et al. Increased binding affinity enhances targeting of glioma xenografts by EGFRvIII-specific scFv. Int J Cancer 2000 Dec 15; 88 (6): 962–9
Humphrey PA, Wong AJ, Vogelstein B, et al. Anti-synthetic peptide antibody reacting at the fusion junction of deletion-mutant epidermal growth factor receptors in human glioblastoma. Proc Natl Acad Sci U S A 1990 Jun; 87 (11): 4207–11
Beers R, Chowdhury P, Bigner D, et al. Immunotoxins with increased activity against epidermal growth factor receptor vIII-expressing cells produced by antibody phage display. Clin Cancer Res 2000 Jul; 6 (7): 2835–43
Sampson JH, Akabani G, Archer GE, et al. Progress report of a phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol 2003 Oct; 65 (1): 27–35
Sampson JH, Reardon DA, Friedman AH, et al. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: case study. Neuro Oncol 2005 Jan; 7 (1): 90–6
Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol 2008 Jun; 10 (3): 320–9
Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 2001 Feb; 61 (3): 842–7
Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res 2005 Aug; 11 (15): 5515–25
Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res 2008 Jul; 68 (14): 5955–64
De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008 May; 14 (10): 3098–104
Mackensen A, Herbst B, Chen JL, et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34 (+) hematopoietic progenitor cells. Int J Cancer 2000 May; 86 (3): 385–92
Caruso DA, Orme LM, Neale AM, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro Oncol 2004 Jul; 6 (3): 236–46
Mitchell DA, Cui X, Schmittling RJ, et al. Monoclonal antibody blockade of IL-2 receptor a during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood 2011 Sep; 118 (11): 3003–12
Sampson JH, Archer GE, Mitchell DA, et al. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol 2008 Oct; 20 (5): 267–75
Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010 Nov; 28 (31): 4722–9
Schmittling RJ, Archer GE, Mitchell DA, et al. Detection of humoral response in patients with glioblastoma receiving EGFRvIII-KLH vaccines. J Immunol Methods 2008 Nov; 339 (1): 74–81
Pfizer Inc. Notice of 2011 Annual Meeting of Shareholders and Proxy Statement. 2011 Mar 22 [online]. Available from URL: http://www.pfizer.com/files/annualreport/2010/proxy/proxyfinancial2010.pdf [Accessed 2012 Feb 1]
Hartmann JT, Haap M, Kopp HG, et al. Tyrosine kinase inhibitors: a review on pharmacology, metabolism and side effects. Curr Drug Metab 2009 Jun; 10 (5): 470–81
Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 2009 Jan; 9 (1): 28–39
El-Rayes BF, LoRusso PM. Targeting the epidermal growth factor receptor. Br J Cancer 2004 Aug; 91 (3): 418–24
Giaccone G, Wang Y. Strategies for overcoming resistance to EGFR family tyrosine kinase inhibitors. Cancer Treat Rev 2011 Oct; 37 (6): 456–64
Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 2003 Jun; 21 (12): 2237–46
Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003 Oct; 290 (16): 2149–58
Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005; 366 (9496): 1527–37
Comis RL. The current situation: erlotinib (Tarceva) and gefitinib (Iressa) in non-small cell lung cancer. Oncologist 2005 Aug; 10 (7): 467–70
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009 Sep; 361 (10): 947–57
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004 May; 350 (21): 2129–39
Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004 Jun; 304 (5676): 1497–500
Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 1. J Clin Oncol 2004 Mar; 22 (5): 777–84
Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 2. J Clin Oncol 2004 Mar; 22 (5): 785–94
Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected]. J Clin Oncol 2009 Apr; 27 (11): 1864–71
Vieitez JM, Valladares M, Pelaez I, et al. A randomized phase II study of raltitrexed and gefitinib versus raltitrexed alone as second line chemotherapy in patients with colorectal cancer. (1839IL/0143). Invest New Drugs 2011 Oct; 29 (5): 1038–44
Ciardiello F, Troiani T, Caputo F, et al. Phase II study of gefitinib in combination with docetaxel as first-line therapy in metastatic breast cancer. Br J Cancer 2006 Jun; 94 (11): 1604–9
Dennison SK, Jacobs SA, Wilson JW, et al. A phase II clinical trial of ZD1839 (Iressa) in combination with docetaxel as first-line treatment in patients with advanced breast cancer. Invest New Drugs 2007 Dec; 25 (6): 545–51
Baselga J, Albanell J, Ruiz A, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol 2005 Aug; 23 (23): 5323–33
Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res 2010 Mar; 16 (6): 1904–14
Bernsdorf M, Ingvar C, Jorgensen L, et al. Effect of adding gefitinib to neo-adjuvant chemotherapy in estrogen receptor negative early breast cancer in a randomized phase II trial. Breast Cancer Res Treat 2011 Apr; 126 (2): 463–70
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005 Jul; 353 (2): 123–32
Tran HT, Zinner RG, Blumenschein Jr GR, et al. Pharmacokinetic study of the phase III, randomized, double-blind, multicenter trial (TRIBUTE) of paclitaxel and carboplatin combined with erlotinib or placebo in patients with advanced Non-small Cell Lung Cancer (NSCLC). Invest New Drugs 2011 Jun; 29 (3): 499–505
Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005 Sep; 23 (25): 5892–9
Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007 Apr; 25 (12): 1545–52
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010 Jun; 11 (6): 521–9
Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011 May; 377 (9780): 1846–54
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007 May; 25 (15): 1960–6
Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res 2004 Sep; 64 (18): 6652–9
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New Engl J Med 2006 Dec; 355 (26): 2733–43
Burris 3rd HA. Dual kinase inhibition in the treatment of breast cancer: initial experience with the EGFR/ErbB-2 inhibitor lapatinib. Oncologist 2004; 9Suppl. 3: 10–5
Castellino S, O’Mara M, Koch K, et al. Human metabolism of lapatinib, a dual kinase inhibitor: implications for hepatotoxicity. Drug Metab Dispos 2012 Jan; 40 (1): 139–50
Wong KK, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res 2009 Apr; 15 (7): 2552–8
Sequist LV, Besse B, Lynch TJ, et al. Neratinib, an irreversible pan-ErbB receptor tyrosine kinase inhibitor: results of a phase II trial in patients with advanced non-small-cell lung cancer. J Clin Oncol 2010 Jun; 28 (18): 3076–83
Metro G, Crino L. The LUX-Lung clinical trial program of afatinib for non-small-cell lung cancer. Expert Rev Anticancer Ther 2011 May; 11 (5): 673–82
Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Semin Oncol 2001 Oct; 28 (5 Suppl. 16): 80–5
Rixe O, Franco SX, Yardley DA, et al. A randomized, phase II, dose-finding study of the pan-ErbB receptor tyrosine-kinase inhibitor CI-1033 in patients with pretreated metastatic breast cancer. Cancer Chemother Pharmacol 2009 Nov; 64 (6): 1139–48
Janne PA, von Pawel J, Cohen RB, et al. Multicenter, randomized, phase II trial of CI-1033, an irreversible pan-ERBB inhibitor, for previously treated advanced non small-cell lung cancer. J Clin Oncol 2007 Sep; 25 (25): 3936–44
Campos S, Hamid O, Seiden MV, et al. Multicenter, randomized phase II trial of oral CI-1033 for previously treated advanced ovarian cancer. J Clin Oncol 2005 Aug; 23 (24): 5597–604
Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res 2002 Dec; 62 (24): 7284–90
Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res 2002 Aug; 62 (16): 4645–55
Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res 2003 Apr; 9 (4): 1546–56
Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 2010 Jul; 11 (7): 619–26
Natale RB, Thongprasert S, Greco FA, et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011 Mar; 29 (8): 1059–66
de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. J Clin Oncol 2011 Mar; 29 (8): 1067–74
Soria JC, Cortes J, Massard C, et al. Phase I safety, pharmacokinetic and pharmacodynamic trial of BMS-599626 (AC480), an oral pan-HER receptor tyrosine kinase inhibitor, in patients with advanced solid tumors. Ann Oncol 2012 Feb; 23 (2): 463–71
Wong NS, Fernando NH, Nixon AB, et al. A phase II study of capecitabine, oxaliplatin, bevacizumab and cetuximab in the treatment of metastatic colorectal cancer. Anticancer Res 2011 Jan; 31 (1): 255–61
Spigel DR, Greco FA, Waterhouse D, et al. Phase II trial of FOLFOX6, bevacizumab, and cetuximab in the first-line treatment of metastatic colorectal cancer. Clin Adv Hematol Oncol 2010 Jul; 8 (7): 480–5, 98
Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells — perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006 Oct; 66 (19): 9339–44
Van Meir EG, Hadjipanayis CG, Norden AD, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 2010 May–Jun; 60 (3): 166–93
Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol 2009 Feb; 27 (2): 129–39
Salemi S, D’Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol 2010 Jun; 29 (3): 247–69
Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 2005 Aug; 54 (8): 721–8
Khoruts A, Fraser JM. A causal link between lymphopenia and autoimmunity. Immunol Lett 2005 Apr; 98 (1): 23–31
Krupica Jr T, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: a two-hit model. Clin Immunol 2006 Aug; 120 (2): 121–8
Acknowledgments
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
The authors would like to thank Marie-Thérése Stockhausen for her editorial support in the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nedergaard, M.K., Hedegaard, C.J. & Poulsen, H.S. Targeting the Epidermal Growth Factor Receptor in Solid Tumor Malignancies. BioDrugs 26, 83–99 (2012). https://doi.org/10.2165/11599760-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11599760-000000000-00000