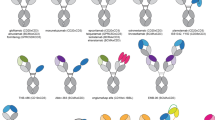
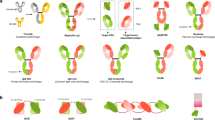
Abstract
Bispecific antibodies (BiAbs) offer a unique opportunity to redirect immune effector cells to kill cancer cells. BiAbs combine the benefits of different binding specificities of two monoclonal antibodies (mAbs) into a single construct. This unique feature of BiAbs enables approaches that are not possible with single mAbs. Advances in antibody engineering and antigen profiling of malignant cells have led to the development of a number of BiAb formats and their combinations for redirecting effector cells to tumor targets. There have been significant advances in the design and application of BiAbs for intravenous and local injection.The initial barrier of cytokine storm has been partially overcome by more recent constructs that have improved clinical effectiveness without dose-limiting toxicities. Since the recent revival of BiAbs, there has been multiple, ongoing, phase I/II and III trials, and some promising clinical outcomes have been reported in completed clinical studies. This review focuses on arming T cells with BiAbs to create the ‘poor man’s cytotoxic lymphocyte’.





Similar content being viewed by others
References
Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer 2002 Aug 20; 100 (6): 690–7
Asano R, Ikoma K, Kawaguchi H, et al. Application of the Fc fusion format to generate tag-free bi-specific diabodies. FEBS J 2010; 277 (2): 477–87
Asano R, Watanabe Y, Kawaguchi H, et al. Highly effective recombinant format of a humanized IgG-like bispecific antibody for cancer immunotherapy with retargeting of lymphocytes to tumorcells. J Biol Chem 2007 Sep 21; 282 (38): 27659–65
Rossi EA, Goldenberg DM, Cardillo TM, et al. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A 2006; 103 (18): 6841–6
Cao Y, Lam L. Bispecific antibody conjugates in therapeutics. Adv Drug Deliv Rev 2003 Feb 10; 55 (2): 171–97
Segal DM, Weiner GJ, Weiner LM. Introduction: bispecific antibodies. J Immunol Methods 2001 Feb 1; 248 (1–2): 1–6
Grimm EA, Mazumder A, Zhang HZ, et al. Lymphokine-activated killer cell phenomenon: lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982; 155: 1823–41
Anderson PM, Bach FH, Ochoa AC. Augmentation of cell number and LAK activity in peripheral blood mononuclear cells activated with anti-CD3 and interleukin-2: preliminary results in children with acute lymphocytic leukemia and neuroblastoma. Cancer Immunol Immunother 1988; 27: 82–8
Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 233: 1318–21
Uberti JP, Joshi I, Ueda M, et al. Preclinical studies using immobilized OKT3 to activate human T cells for adoptive immunotherapy: optimal conditions for the proliferation and induction of non-MHC restricted cytotoxicity. Clin Immunol Immunopathol 1994; 70: 234–40
Ueda M, Joshi ID, Dan M, et al. Preclinical studies for adoptive immunotherapy in bone marrow transplantation, II: generation of anti-CD3 activated cytotoxic T cells from normal donors and autologous bone marrow transplant candidates. Transplantation 1993; 56: 351–6
Lum LG, LeFever AV, Treisman JS, et al. Immune modulation in cancer patients after adoptive transfer of anti-CD3/anti-CD28-costimulated T cells: phase I clinical trial. J Immunother 2001 Sep; 24 (5): 408–19
Fowler DH, Odom J, Steinberg SM, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2006 Nov; 12 (11): 1150–60
Garlie NK, LeFever AV, Siebenlist RE, et al. T cells co-activated with immobilized anti-CD3 and anti-CD28 as potential immunotherapy for cancer. J Immunother 1999; 4: 335–45
Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. N Engl J Med 1988; 319: 1676–80
Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and auto-immunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002 Oct 25; 298 (5594): 850–4
Whiteside TL. Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol Immunother 1999; 48: 346–52
Weiden PL, Sullivan KM, Flournoy N, et al. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med 1981 Jun 18; 304 (25): 1529–33
Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev 2010 Nov; 36 (7): 528–38
Kolb HJ, Mittermuller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990; 76: 2462–5
Kolb HJ, Holler E. Adoptive immunotherapy with donor lymphocyte transfusions. Curr Opin Oncol 1997; 9: 139–45
Liu Z, Savoldo B, Huls H, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the prevention and treatment of EBV-associated post-transplant lymphomas. Recent Results Cancer Res 2002; 159: 123–33
Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998 Sep 1; 92 (5): 1549–55
Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010 Feb 4; 115 (5): 925–35
Rivoltini L, Barracchini KC, Viggiano V, et al. Quantitative correlation between HLA class I allele expression and recognition of melanoma cells by antigen-specific cytotoxic T lymphocytes. Cancer Res 1995 Jul 15; 55 (14): 3149–57
Cohen SB, Katsikis PD, Feldmann M, et al. IL-10 enhances expression of the IL-2 receptor alpha chain on T cells. Immunology 1994 Nov; 83 (3): 329–32
Concha A, Cabrera T, Ruiz-Cabello F, et al. Can the HLA phenotype be used as a prognostic factor in breast carcinomas? Int J Cancer Suppl 1991; 6: 146–54
Blades RA, Keating PJ, McWilliam LJ, et al. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology 1995 Nov; 46 (5): 681–6
Browning M, Petronzelli F, Bicknell D, et al. Mechanisms of loss of HLA class I expression on colorectal tumor cells. Tissue Antigens 1996 May; 47 (5): 364–71
Redondo M, Concha A, Oldiviela R, et al. Expression of HLA class I and II antigens in bronchogenic carcinomas: its relationship to cellular DNA content and clinical-pathological parameters. Cancer Res 1991 Sep 15; 51 (18): 4948–54
Torres MJ, Ruiz-Cabello F, Skoudy A, et al. Loss of an HLA haplotype in pancreas cancer tissue and its corresponding tumor derived cell line. Tissue Antigens 1996 May; 47 (5): 372–81
Ferrone S, Marincola FM. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today 1995 Oct; 16 (10): 487–94
Smyth MJ, Strobl SL, Young HA, et al. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes: inhibition by transforming growth factor-β. J Immunol 1991; 146: 3289–97
Blay JY, Negrier S, Combaret V, et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res 1992 Jun 15; 52 (12): 3317–22
Tartour E, Blay JY, Dorval T, et al. Predictors of clinical response to interleukin-2-based immunotherapy in melanoma patients: a French multi-institutional study. J Clin Oncol 1996 May; 14 (5): 1697–703
Nitta T, Sato K, Yagita H, et al. Preliminary trial of specific targeting therapy against malignant glioma. Lancet 1990; 335: 368–71
Bolhuis RL, Lamers CH, Goey SH, et al. Adoptive immunotherapy of ovarian carcinoma with bs-mAb-targeted lymphocytes: a multicenter study. Int J Cancer Suppl 1992; 7: 78–81
de Gast GC, van Houten AA, Haagen IA, et al. Clinical experience with CD3 × CD19 bispecific antibodies in patients with B cell malignancies. J Hematother 1995 Oct; 4 (5): 433–7
Kroesen BJ, Nieken J, Sleijfer DT, et al. Approaches to lung cancer treatment using the CD3 × EGP-2-directed bispecific monoclonal antibody BIS-1. Cancer Immunol Immunother 1997 Nov; 45 (3–4): 203–6
Hartmann F, Renner C, Jung W, et al. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Blood 1997; 89 (6): 2042–7
Chen J, Bashey A, Holman P, et al. A phase I dose escalating study of infusion of a bispecific antibody (BsAb) for relapsed/refractory acute myeloid leukemia (AML) [abstract]. Blood 1999 Nov 15; 94 (10): 227B
James ND, Atherton PJ, Jones J, et al. A phase II study of the bispecific antibody MDX-H210 (anti-HER2 × CD64) with GM-CSF in HER2+ advanced prostate cancer. Br J Cancer 2001; 85 (2): 152–6
Valone FH, Kaufman PA, Guyre PM, et al. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol 1995; 13 (9): 2281–92
Burges A, Wimberger P, Kumper C, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM × anti-CD3 antibody: a phase I/II study. Clin Cancer Res 2007; 13 (13): 3899–905
Sebastian M, Passlick B, Friccius-Quecke H, et al. Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM × anti-CD3): a phase I study. Cancer Immunol Immunother 2007; 56 (10): 1637–44
Borghaei H, Alpaugh RK, Bernardo P, et al. Induction of adaptive Anti-HER2/neu immune responses in a Phase 1B/2 trial of 2B1 bispecific murine monoclonal antibody in metastatic breast cancer (E3194): a trial coordinated by the Eastern Cooperative Oncology Group. J Immunother 2007 May; 30 (4): 455–67
Weiner LM, Clark JI, Davey M, et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer Res 1995; 55 (20): 4586–93
Fury MG, Lipton A, Smith KM, et al. A phase-I trial of the epidermal growth factor receptor directed bispecific antibody MDX-447 without and with recombinant human granulocyte-colony stimulating factor in patients with advanced solid tumors. Cancer Immunol Immunother 2008 Feb; 57 (2): 155–63
Barbara Ann Karmanos Cancer Institute. Donor T cells, low-dose aldesleukin, and low-dose GM-CSF after donor stem cell transplant in treating patients with relapsed or refractory non-Hodgkin’s lymphoma [Clinical-Trials.gov identifier NCT00521261]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
Barbara Ann Karmanos Cancer Institute. Laboratory-treated T cells after second-line chemotherapy in treating women with HER2/neu-negative metastatic breast cancer [ClinicalTrials.gov identifier NCT01022138]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
AGO Study Group. Randomized, multicenter, 2-dose level. open-label, phase IIa study with the intraperitoneally infused trifunctional bispecific antibody Removab™ (anti-EpCAM × anti-CD3) to select the better dose level in platinum refractory epithelial ovarian cancer patients [ClinicalTrials.gov identifier NCT00189345]. US National Institutes of Health, Clinical-Trials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
Fresenius Biotech GmbH. Phase II study with the trifunctional antibody ertumaxomab to treat metastatic breast cancer progressing after endocrine treatment [ClinicalTrials.gov identifier NCT00452140]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
University of Tuebingen (Germany). Phase I/II study with local treatment of metastatic melanoma with autologous lymphocytes and the bispecific antibody rM28 [ClinicalTrials.gov identifier: NCT00204594]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
Micromet AG. Safety study of the bispecific t-cell engager blinatumomab (MT103) in patients with relapsed NHL [ClinicalTrials.gov identifier NCT00274742]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
Micromet AG. Phase II study of the BiTE® Blinatumomab (MT103) in patients with minimal residual disease of B-precursor acute ALL [ClinicalTrials.gov identifier NCT00560794]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov [Accessed 2011 Sep 8]
Raso V, Griffin T. Hybrid antibody with dual specificity for the delivery of ricin to immunoglobin bearing target cells. Cancer Res 1981; 41: 2073–8
Titus JA, Perez P, Kaubisch A, et al. Human K/natural killer cells targeted with hetero-cross-linked antibodies specifically lyse tumor cells in vitro and prevent tumor growth in vivo. J Immunol 1987; 139: 3153–8
Perez P, Hoffman RW, Shaw S. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature 1985; 316: 354–6
Kosterink JG, de Jonge MW, Smit EF, et al. Pharmacokinetics and scintigraphy of indium-111-DTPA-MOC-31 in small-cell lung carcinoma. J Nucl Med 1995 Dec; 36 (12): 2356–62
Weiner LM, Clark JI, Davey M, et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer Res 1995 Oct 15; 55 (20): 4586–93
Hartmann F, Renner C, Jung W, et al. Treatment of refractory Hodgkin’s disease with an anti-CD16/Cd30 bispecific antibody [published erratum appears in Blood 1998, 91: 1832]. Blood 1997; 89: 2042–7
Valone FH, Kaufman PA, Guyre PM, et al. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J Clin Oncol 1995; 13: 2281–92
Schwaab T, Lewis LD, Cole BF, et al. Phase I pilot trial of the bispecific antibody MDXH210 (anti-Fc gamma RI X anti-HER-2/neu) in patients whose prostate cancer overexpresses HER-2/neu. J Immunother 2001 Jan; 24 (1): 79–87
James ND, Atherton PJ, Jones J, et al. A phase II study of the bispecific antibody MDX-H210 (anti-HER2 × CD64) with GM-CSF in HER2+ advanced prostate cancer. Br J Cancer 2001 Jul 20; 85 (2): 152–6
Posey JA, Raspet R, Verma U, et al. A pilot trial of GM-CSF and MDX-H210 in patients with erbB-2-positive advanced malignancies. J Immunother 1999 Jul; 22 (4): 371–9
Borchmann P, Schnell R, Fuss I, et al. Phase 1 trial of the novel bispecific molecule H22xKi-4 in patients with refractory Hodgkin lymphoma. Blood 2002 Nov 1; 100 (9): 3101–7
Zeidler R, Reisbach G, Wollenberg B, et al. Simultaneous activation of T cells and accessory cells by a new class of intact bispecific antibody results in efficient tumor cell killing. J Immunol 1999; 163 (3): 1246–52
Zeidler R, Mysliwietz J, Csanady M, et al. The Fc-region of a new class of intact bispecific antibody mediates activation of accessory cells and NK cells and induces direct phagocytosis of tumour cells. Br J Cancer 2000 Jul; 83 (2): 261–6
Kiewe P, Hasmuller S, Kahlert S, et al. Phase I trial of the trifunctional anti-HER2 × anti-CD3 antibody ertumaxomab in metastatic breast cancer. Clin Cancer Res 2006; 12 (10): 3085–91
Baeuerle PA, Kufer P, Lutterbuse R. Bispecific antibodies for polyclonal T-cell engagement. Curr Opin Mol Ther 2003; 5 (4): 413–9
Brischwein K, Parr L, Pflanz S, et al. Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother 2007; 30 (8): 798–807
Dreier T, Lorenczewski G, Brandl C, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer 2002; 100 (6): 690–7
Dreier T, Baeuerle PA, Fichtner I, et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3-bispecific single-chain antibody construct. J Immunol 2003; 170 (8): 4397–402
Loffler A, Kufer P, Lutterbuse R, et al. A recombinant bispecific single-chain antibody, CD19 × CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000; 95 (6): 2098–103
Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321 (5891): 974–7
Topp MS. Treatment with anti-CD19 BiTE antibody blinatumomab (MT103 /MEDI-538) is able to eliminate minimal residual disease (MRD) in patients with B-precursor acute lymphoblastic leukemia (ALL): first results of an ongoing phase II study [abstract]. Blood 2008; 112 Suppl.: 1926
Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 2009; 69 (12): 4941–4
Baeuerle PA, Kufer P, Bargou R. BiTE: teaching antibodies to engage T-cells for cancer therapy. Curr Opin Molecular Ther 2009 Feb; 11 (1): 22–30
Lutterbuese R, Raum T, Kischel R, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci USA 2010 Jul 13; 107 (28): 12605–10
Schlereth B, Fichtner I, Lorenczewski G, et al. Eradication of tumors from a human colon cancer cell line and from ovarian cancer metastases in immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific antibody construct. Cancer Res 2005 Apr 1; 65 (7): 2882–9
Perez P, Titus JA, Lotze MT, et al. Specific lysis of human tumor cells byT cells coated with anti-T3 cross-linked to anti-tumor antibody. J Immunol 1986; 137 (7): 2069–72
Segal DM, Garrido MA, Perez P, et al. Targeted cytotoxic cells as a novel form of cancer immunotherapy. Mol Immunol 1988; 25: 1099–103
Renner C, Held G, Ohnesorge S, et al. Role of perforin, granzymes and the proliferative state of the target cells in apoptosis and necrosis mediated by bispecific-antibody-activated cytotoxic T cells. Cancer Immunol Immunother 1997; 44: 70–6
Jung G, Brandl M, Eisner W, et al. Local immunotherapy of glioma patients with a combination of 2 bispecific antibody fragments and resting autologous lymphocytes: evidence for in situ t-cell activation and therapeutic efficacy. Int J Cancer 2001 Jan 15; 91 (2): 225–30
Lamers CHJ, van de Griend RJ, Braakman E, et al. Optimization of culture conditions for activation and large-scale expansion of human T lymphocytes for bispecific antibody-directed cellular immunotherapy. Int J Cancer 1992; 51: 973–9
Lamers CH, Bolhuis RL, Warnaar SO, et al. Local but no systemic immunomodulation by intraperitoneal treatment of advanced ovarian cancer with autologous T lymphocytes re-targeted by a bi-specific monoclonal antibody. Int J Cancer 1997 Oct 9; 73 (2): 211–9
Canevari S, Stoter G, Arienti F, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst 1995; 87: 1463–9
Sen M, Wankowski DM, Garlie NK, et al. Use of anti-CD3 × anti-HER2/neu bispecific antibody for redirecting cytotoxicity of activated T cells toward HER2/neu tumors. J Hematother Stem Cell Res 2001 Apr; 10: 247–60
Lum HE, Miller M, Davol PA, et al. Preclinical studies comparing different bispecific antibodies for redirecting T cell cytotoxicity to extracellular antigens on prostate carcinomas. Anticancer Res 2005 Jan; 25 (1A): 43–52
Chan JK, Hamilton CA, Cheung MK, et al. Enhanced killing of primary ovarian cancer by retargeting autologous cytokine-induced killer cells with bispecific antibodies: a preclinical study. Clin Cancer Res 2006 Mar 15; 12 (6): 1859–67
Lum LG, Davol P, Grabert R, et al. Targeting pancreatic cancer with armed activated T cells directed at Her2/neu receptors [abstract]. Exp Hematol 2002; 30: 56
Grabert RC, Smith J, Tiggs J, et al. Anti-CD3 activated T cells armed with OKT3 × herceptin bispecific antibody, survive and divide, and secrete cytokines and chemokines after multiple cycles of killing directed at Her2/neu+ tumor targets [abstract]. Am Assoc Cancer Res 2003; 44: 656a
Davol PA, Smith JA, Kouttab N, et al. Anti-CD3 × Anti-HER2 bispecific antibody effectively redirects armed T cells to inhibit tumor development and growth in hormone-refractory prostate cancer-bearing SCID-Beige mice. Clin Prostate Cancer 2004 Sep; 3: 112–21
Kroesen BJ, ter Haar A, Willemse P, et al. Local antitumor treatment in carcinoma patients with bispecific-monoclonal-antibody-redirected T cells. Cancer Immunol Immunother 1993; 37 (6): 401–7
Demanet C, Brissinck J, De Jong J, et al. Bispecific antibody-mediated immunotherapy of the BCL1 lymphoma: increased efficacy with multiple injections and CD28-induced costimulation. Blood 1996; 87: 4390–8
Hombach A, Tillmann T, Jensen M, et al. Specific activation of resting T cells against CA19-9+ tumor cells by an anti-CD3/CA19-9 bispecific antibody in combination with a costimulatory anti-CD28 antibody. J Immunother 1997 Sep; 20 (5): 325–33
Kaneko T, Fusauchi Y, Kakui Y, et al. A bispecific antibody enhances cytokine-induced killer-mediated cytolysis of autologous acute myeloid leukemia cells. Blood 1993; 81 (5): 1333–41
Bohlen H, Hopff T, Manzke O, et al. Lysis of malignant B cells from patients with B-chronic lymphocytic leukemia by autologous T cells activated with CD3 × CD19 bispecific antibodies in combination with bivalent CD28 antibodies. Blood 1993; 82: 1803–12
Bohlen H, Manzke O, Patel B, et al. Cytolysis of leukemic B-cells by T-cells activated via two bispecific antibodies. Cancer Res 1993; 43: 4310–4
Klein SC, Boer LH, de Weger RA, et al. Release of cytokines and soluble cell surface molecules by PBMC after activation with the bispecific antibody CD3 × CD19. Scand J Immunol 1997; 46: 452–8
Anderson PM, Crist W, Hasz D, et al. G19.4 (αCD3) × B43 (αCD19) monoclonal antibody heteroconjugate triggers CD19 antigen-specific lysis of t (4;11) acute lymphoblastic leukemia cells by activated CD3 antigen-positive cytotoxic T cells. Blood1992; 80 (11): 2826–34
Bejeck BE, Wang D, Berven E, et al. Development and characterization of three recombinant single chain antibody fragments (scFvs) directed against the CD19 antigen. Cancer Res 1995; 55: 2346–51
de Gast GC, Haagen I-A, van Houten AA, et al. CD8 T cell activation after intravenous administration of CD3 × CD19 bispecific antibody in patients with non-Hodgkin lymphoma. Cancer Immunol Immunother 1995; 40: 390–6
Gall JM, Davol PA, Grabert RC, et al. T cells armed with anti-CD3 × anti-CD20 bispecific antibody enhance killing of CD20+ malignant B cells and bypass complement-mediated rituximab resistance in vitro. Exp Hematol 2005 Apr; 33 (4): 452–9
Renner C, Jung W, Sahin U, et al. Cure of xenografted human tumors by bispecific monoclonal antibodies and human T cells. Science 1994; 264: 833–5
Renner C, Bauer S, Sahin U, et al. Cure of disseminated xenografted human Hodgkin’s tumors by bispecifc monoclonal antibodies and human T cells: the role of human T-cell subsets in a preclinical model. Blood 1996; 87 (7): 2930–7
Pohl C, Denfeld R, Renner C, et al. CD30-antigen-specific targeting and activation of T cells via murine bispecific monoclonal antibodies against CD3 and CD28: potential use for the treatment of Hodgkin’s lymphoma. Int J Cancer 1993; 54: 820–7
Kuwahara M, Kuroki M, Arakawa F, et al. A mouse/human-chimeric bispecific antibody reactive with human carcinoembryonic antigen-expressing cells and human T-lymphocytes. Anticancer Res 1997; 16: 2661–8
Negri DR, Tosi E, Valota O, et al. In vitro and in vivo stability and anti-tumour efficacy of an anti-EGFR/anti-CD3 F (ab’)2 bispecific monoclonal antibody. Br J Cancer 1995 Oct; 72 (4): 928–33
Reusch U, Sundaram M, Davol PA, et al. Anti-CD3 × anti-EGFR bispecific antibody redirects T cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin Cancer Res 2006; 12: 183–90
Riesenberg R, Buchner A, Pohla H, et al. Lysis of prostate carcinoma cells by trifunctional bispecific antibodies (alpha EpCAM × alpha CD3). J Histochem Cytochem 2001 Jul; 49 (7): 911–7
Luiten RM, Coney LR, Fleuren GJ, et al. Generation of chimeric bispecific G250/anti-CD3 monoclonal antibody, a tool to combat renal cell carcinoma. Br J Cancer 1996 Sep; 74 (5): 735–44
Nitta T, Sato K, Okumura K, et al. Induction of cytotoxicity in human T cells coated with anti-glioma × anti-CD3 bispecific antibody against human glioma cells. J Neurosurg 1990; 72: 476–81
Shalaby MR, Shepard HM, Presta L, et al. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J Exp Med 1992; 175: 217–25
Shalaby MR, Carter P, Maneval D, et al. Bispecific Her2 × CD3 antibodies enhance T-cell cytotoxicity in vitro and localize to Her2-overexpressing xenograpfts in nude mice. Clin Immunol Immunopathol 1995; 74: 185–92
Brossart P, Stuhler G, Flad T, et al. Her-2/neu-derived peptides are tumor-associated antigens expressed by human renal cell and colon carcinoma lines and are recognized by in vitro induced specific cytotoxic T lymphocytes. Cancer Res 1998; 58: 732–6
Davol PA, Smith JA, Kouttab N, et al. Anti-CD3 × anti-HER2 bispecific antibody effectively redirects armed T cells to inhibit tumor development and growth in hormone-refractory prostate cancer-bearing severe combined immunodeficient beige mice. Clin Prostate Cancer 2004 Sep; 3 (2): 112–21
Zhu Z, Lewis GD, Carter P. Engineering high affinity humanized anti-p185HER2/anti-CD3 bispecific F (ab’)2 for efficient lysis of p185HER2 overexpressing tumor cells. Int J Cancer 1995; 62: 319–24
Kostelny SA, Link BK, Tso JY, et al. Humanization and characterization of the anti-HLA-DR antibody 1D10. Int J Cancer 2001 Aug 15; 93 (4): 556–65
Zhu Z, Ghose T, Lee SH, et al. Tumor localization and therapeutic potential of an antitumor-anti-CD3-heteroconjugate antibody in human renal cell carcinoma xenograft models. Cancer Lett 1994 Oct 28; 86 (1): 127–34
Katayose Y, Kudo T, Suzuki M, et al. MUC1-specific targeting immunotherapy with bispecific antibodies: inhibition of xenografted human bile duct carcinoma growth. Cancer Res 1996; 56: 4205–12
Katzenwadel A, Schleer H, Gierschner D, et al. Construction and in vivo evaluation of an anti-PSA × anti-CD3 bispecific antibody for the immunotherapy of prostate cancer. Anticancer Res 2000 May; 20 (3A): 1551–5
Davico BL, De Monte LB, Spagnoli GC, et al. Bispecific monoclonal antibody anti-CD3 × anti-tenascin: an immunotherapeutic agent for human glioma. Int J Cancer 1995 May 16; 61 (4): 509–15
Jost CR, Titus JA, Kurucz I, et al. A single-chain bispecific Fv2 molecule produced in mammalian cells redirects lysis by activated CTL. Mol Immunol 1996 Feb; 33 (2): 211–9
Chapoval AI, Nelson H, Thibault C. Anti-CD3 × anti-tumor F (ab’)2 bifunctional antibody activates and retargets tumor-infiltrating lymphocytes. J Immunol 1995; 155: 1296–303
Davol PA, Gall JM, Grabert RC, et al. Infusions of T cells armed with anti-CD3 × anti-her2/neu bispecific antibody modulate in vivo patient immune responses in phase I clinical trials for breast and hormone refractory prostate cancers [abstract]. Blood 2004 Nov 16; 104 (11): 379a
Lum LG, Rathore R, Colvin GA, et al. Targeting HER2/neu tumor cells with anti-CD3 activated T cells: clinical trials and trafficking studies [abstract]. ASCO Meet Proc 2003; 22: 179
Lum LG, Thakur A, Rathore R, et al. Phase I clinical trial involving infusions of activated T cells armed with anti-CD3 × anti-Her2neu bispecific antibody in women with metastatic breast cancer: clinical, immune, and trafficking results. ASCO Breast Cancer Symposium; 2010 Oct 1–3, Washington, DC
Lum LG, Thakur A, Al-Khadimi Z, et al. Phase I dose escalation of activated T cells (ATC) armed with anti-CD3 × anti-CD20 bispecific antibody (CD20Bi) after stem cell transplant (SCT) in non-Hodgkin’s lymphoma (NHL) [abstract]. The American Society of Hematology (ASH) Annual Meeting 2010; 2010 Dec 4–7; Orlando (FL): 488
Grabert RC, Cousens LP, Smith JA, et al. Human T cells armed with Her2/neu bispecific antibodies divide, are cytotoxic, and secrete cytokines with repeated stimulation. Clin Cancer Res 2006; 12 (2): 569–76
Mullbacher A, Lobigs M, Tha Hla R, et al. Antigen-dependent release of IFN-gamma by cytotoxic T cells up-regulates Fas on target cells and facilitates exocytosis-independent specific target cells lysis. J Immunol 2002; 169: 145–50
Zeytun A, Hassuneh M, Nagarkatti M, et al. Fas-Fas ligand-based interactions between tumor cells and tumor-specific cytotoxic T lymphocytes: a lethal two-way street. Blood 1997; 90: 1952–9
Zaks TZ, Chappell DB, Rosenberg SA, et al. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol 1999; 162: 3273–9
Walker PR, Saas P, Dietrich PY. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol 1997 May 15; 158 (10): 4521–4
O’Connell J, O’Sullivan GC, Collins JK, et al. The Fas counterattack: Fasmediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996; 184: 1075–82
Karas M, Zaks TZ, Yakar S, et al. TCR Stimulation protects CD8+ T cells from CD95 mediated apoptosis. Hum Immunol 2001; 62: 32–8
Grosse-Hovest L, Hartlapp I, Marwan W, et al. A recombinant bispecific single-chain antibody induces targeted, supra-agonistic CD28-stimulation and tumor cell killing. Eur J Immunol 2003 May; 33 (5): 1334–40
Otz T, Grosse-Hovest L, Hofmann M, et al. A bispecific single-chain antibody that mediates target cell-restricted, supra-agonistic CD28 stimulation and killing of lymphoma cells. Leukemia 2009 Jan; 23 (1): 71–7
Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006 Sep 7; 355 (10): 1018–28
Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol 1996; 26: 2304–9
Renner C, Jung W, Sahin U, et al. The role of lymphocyte subsets and adhesion molecules in T cell-dependent cytotoxicity mediated by CD3 and CD28 bispecific monoclonal antibodies. Eur J Immunol 1995; 25: 2027–33
Mazzoni A, Mezzanzanica D, Jung G, et al. CD3–CD28 costimulation as a means of avoiding T cell preactivation in bispecific monoclonal antibody-based treatment of ovarian carcinoma. Cancer Res 1996; 56: 5443–9
Hombach A, Tillmann T, Jensen M, et al. Specific activation of resting T cells against tumour cells by bispecific antibodies and CD28-mediated costimulation is accompanied by Th1 differentiation and recruitment of MHC-independent cytotoxicity. Clin Exp Immunol 1997; 108: 352–7
Fitzer-Attas CJ, Eshhar Z. Tyrosine kinase chimeras for antigen-selective T-body therapy. Adv Drug Deliv Rev 1998 Apr 6; 31 (1–2): 171–82
Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA 1993; 90: 720–4
Hwu P, Shafer GE, Treisman JS, et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor gamma chain. J Exp Med 1993; 178: 361–6
Hwu P, Yang JC, Cowherd R, et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res 1995; 55: 3369–73
Fitzer-Attas CJ, Eshhar Z. Tyrosine kinase chimeras for antigen-selective T-body therapy. Adv Drug Delivery Rev 1998; 31: 171–82
Altenschmidt U, Moritz D, Groner B. Specific cytotoxic T lymphocytes in gene therapy. J Mol Med 1997; 75: 259–66
Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer 2003 Jan; 3 (1): 35–45
Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006 Oct 15; 12 (20 Pt 1): 6106–15
Cooper LJ, Al-Kadhimi Z, Serrano LM, et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood 2005 Feb 15; 105 (4): 1622–31
Emtage PC, Lo AS, Gomes EM, et al. Second-generation anticarcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin Cancer Res 2008 Dec 15; 14 (24): 8112–22
Ma Q, Safar M, Holmes E, et al. Anti-prostate specific membrane antigen designer T cells for prostate cancer therapy. Prostate 2004 Sep 15; 61 (1): 12–25
Wang LX, Li R, Yang G, et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res 2005 Nov 15; 65 (22): 10569–77
Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005 Oct 3; 202 (7): 907–12
Grinshtein N, Ventresca M, Margl R, et al. High-dose chemotherapy augments the efficacy of recombinant adenovirus vaccines and improves the therapeutic outcome. Cancer Gene Ther 2009 Apr; 16 (4): 338–50
Salem ML, az-Montero CM, Al-Khami AA, et al. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly (I:C). J Immunol 2009 Feb 15; 182 (4): 2030–40
Curti BD, Longo DL, Ochoa AC, et al. Treatment of cancer patients with ex vivo anti-CD3-activated killer cells and interleukin-2. J Clin Oncol 1993; 11: 652–60
McCall AM, Shahied L, Amoroso AR, et al. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J Immunol 2001 May 15; 166 (10): 6112–7
Haagen IA, de Lau WB, Bast BJ, et al. Unprimed CD4+ and CD8+ T cells can be rapidly activated by a CD3 × CD19 bispecific antibody to proliferate and become cytotoxic. Cancer Immunol Immunother 1994 Dec; 39 (6): 391–6
Acknowledgments
We appreciate the efforts of the physicians, immunotherapy technical staff, clinical coordinators, nursing staff, administrative staff, and the leadership of the Barbara Ann Karmanos Cancer Institute and Roger Williams Medical Center. Special thanks to Wendy Young, Annette Olson, Lori Hall, Patricia Steele, and Karen Myers for their dedicated efforts to serve the immunotherapy patients. The studies were supported by NIH grants R01 CA 092344 (LGL), R01 CA 140314 (LGL); Cancer Center Support Grant P30 CA022453-25; Translational Grants #6092-09 (LGL) and #6066-06 (LGL) from the Leukemia and Lymphoma Society; Susan G. Komen Foundation grant BCTR0707125 (LGL); startup funds from the Karmanos Cancer Institute (LGL); and a gift from the Bill Young Foundation for breast cancer immunotherapy (LGL). LGL is a founder of Transtarget, Inc. AT has no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lum, L.G., Thakur, A. Targeting T Cells with Bispecific Antibodies for Cancer Therapy. BioDrugs 25, 365–379 (2011). https://doi.org/10.2165/11595950-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11595950-000000000-00000